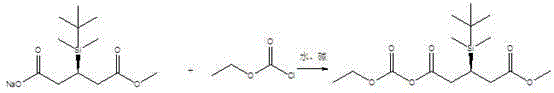
Synthesis of 1-ethoxycarbonyl-5-methyl-(3r)-tert-butyldimethylsilyloxyglutarate
A technology of tert-butyldimethylsiloxyglutarate and sodium tert-butyldimethylsiloxyglutarate is applied in the field of compound synthesis technology and can solve the problem that triethylamine is inflammable, unstable, Explosive and other problems, to achieve the effect of simple raw materials, mild reaction conditions, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] In a 250ml three-necked flask, a thermometer, a pH meter, a constant pressure dropping funnel and a magnetic stirrer were installed. At room temperature, add 50ml of water to the reaction flask, then add 28.2 grams of (3R)-3-tert-butyldimethylsiloxysodium glutarate monomethyl ester, cool down to -5°C, Add 40 grams of 10% by mass sodium hydroxide solution, add 0.282 g of tetrabutylammonium chloride, continue cooling to -5 ° C, dropwise add 10.8 grams of ethyl chloroformate, and stir for 5 hours. Add 50 ml of ethyl acetate for extraction, wash once with 30 ml of saturated brine, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure to obtain 31.8 g of 1-ethoxycarbonyl-5-methyl-(3R)-tert-butyldimethylsilyloxyglutarate (HPLC 98.7%) Yield: 95.8%.
[0013] 1 HNMR (CDCl 3 )δ:0.08(3H,s);0.09(3H,s);0.85(9H,s);3.69(3H,s);1.3-1.4(3H,t,J=7.3Hz);2.5-2.6(d ,2H,d,J=6.3Hz);2.6-2.8(m,2H);4.26-4.38(2H,q,J=7.3Hz);4.5-4.62(m,1H)
Embodiment 2
[0015] In a 250ml three-necked flask, a thermometer, a pH meter, a constant pressure dropping funnel and a magnetic stirrer were installed. At room temperature, add 50ml of water into the reaction flask, then add 28.2 grams of (3R)-3-tert-butyldimethylsiloxysodium glutarate monomethyl ester, cool down to 10°C, and place in a constant pressure dropping funnel Add 18.7 g of 30% by mass potassium hydroxide solution, add 0.0282 g of tetrabutylammonium bromide, continue cooling to 10 ° C, dropwise add 21.6 g of ethyl chloroformate, and stir for 10 hours. Add 50 ml of ethyl acetate for extraction, wash once with 30 ml of saturated brine, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure to obtain 30.1 g of 1-ethoxycarbonyl-5-methyl-(3R)-tert-butyldimethylsilyloxyglutarate (HPLC 98.5%) Yield: 90.8%.
Embodiment 3
[0017] In a 250ml three-necked flask, a thermometer, a pH meter, a constant pressure dropping funnel and a magnetic stirrer were installed. At room temperature, add 50ml of water to the reaction flask, then add 28.2 grams of (3R)-3-tert-butyldimethylsiloxysodium glutarate monomethyl ester, cool down to 0°C, and place in a constant pressure dropping funnel Add 12 grams of 20% by mass lithium hydroxide solution, add 0.1 g of tetrabutylammonium chloride, continue cooling to 0°C, dropwise add 15.0 grams of ethyl chloroformate, and stir for 7 hours. Add 50 ml of ethyl acetate for extraction, wash once with 30 ml of saturated brine, and dry over anhydrous sodium sulfate. Concentrate under reduced pressure to obtain 29.8 g of 1-ethoxycarbonyl-5-methyl-(3R)-tert-butyldimethylsilyloxyglutarate (HPLC 98.6%) yield: 89.7%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap