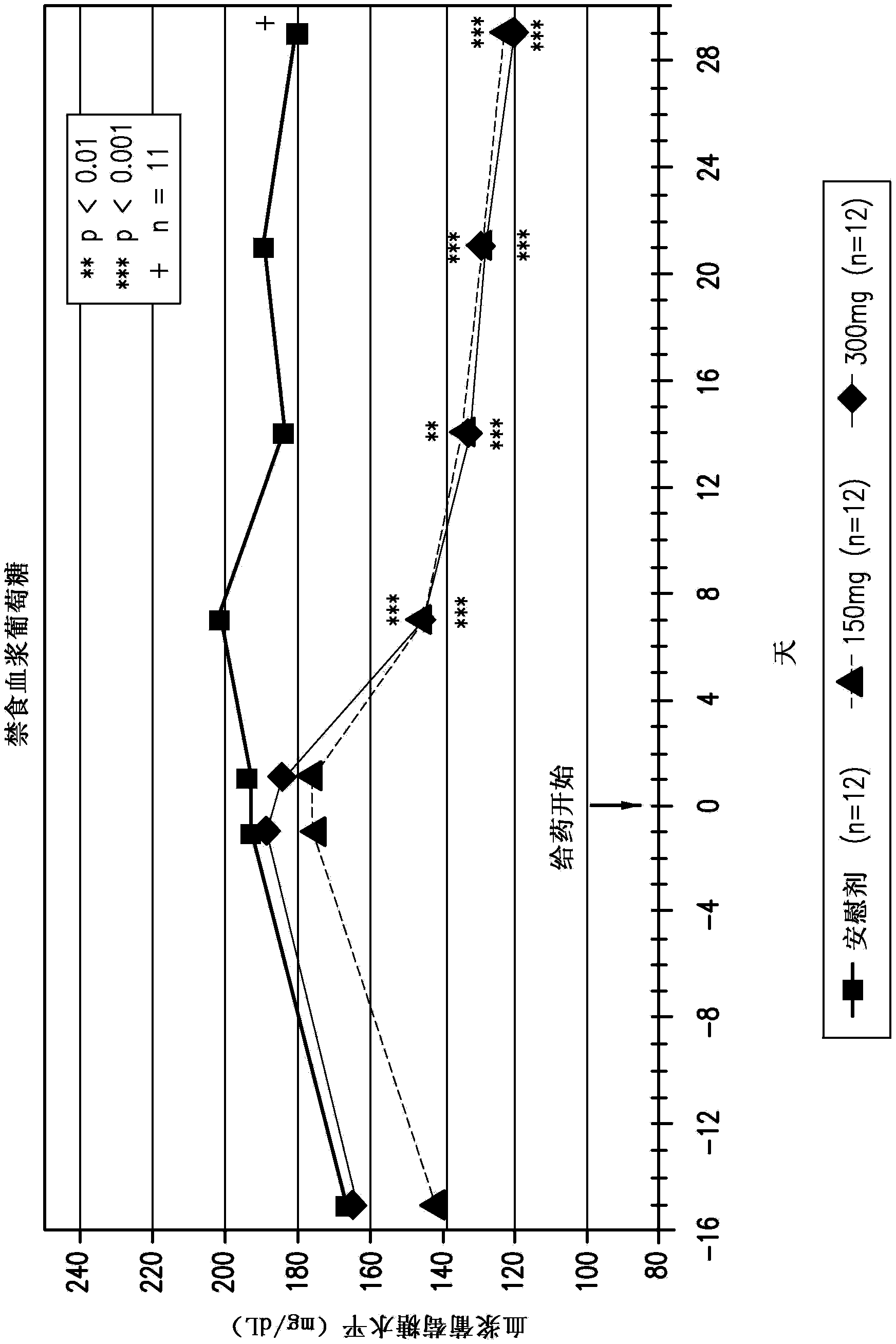
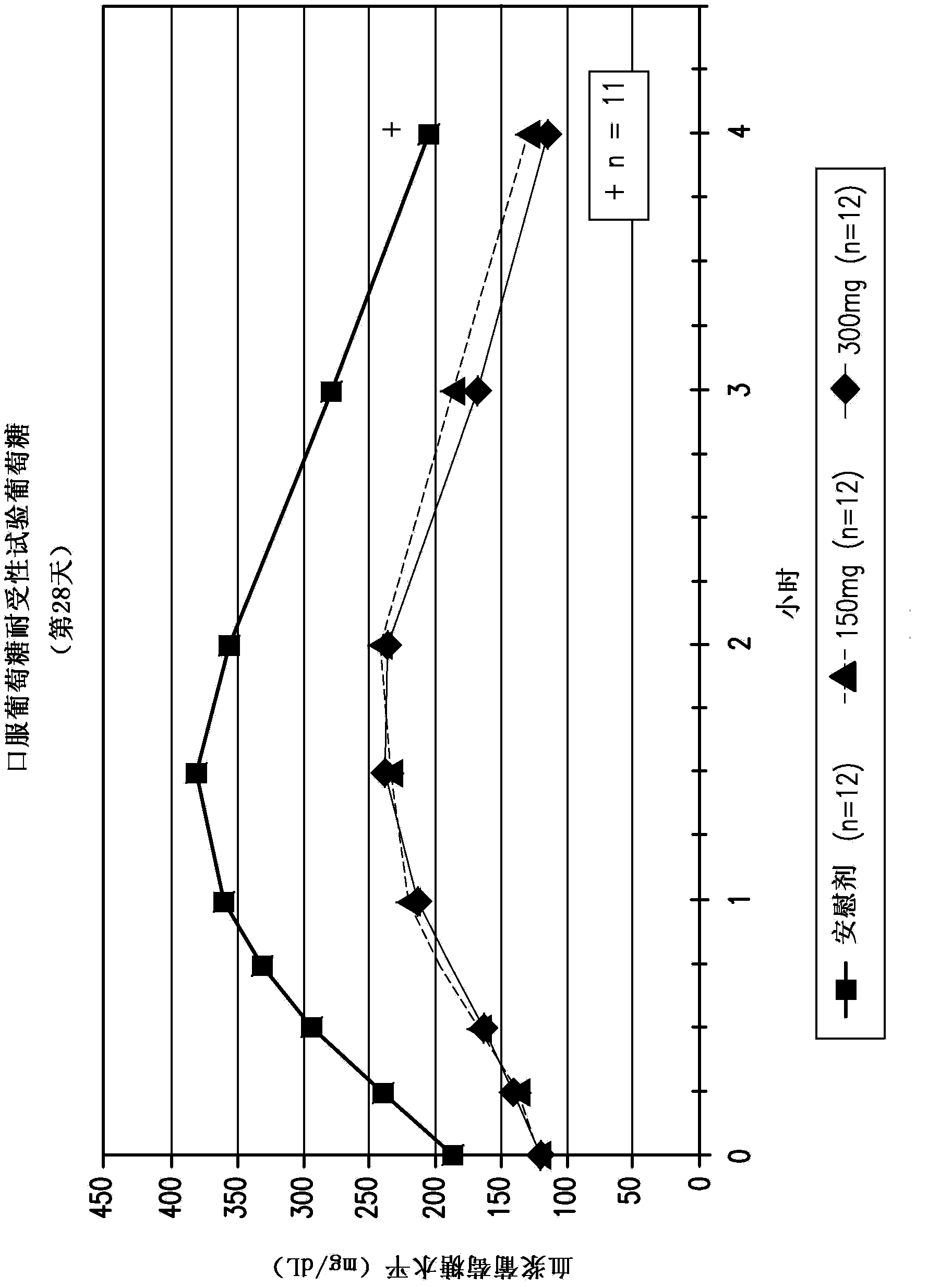
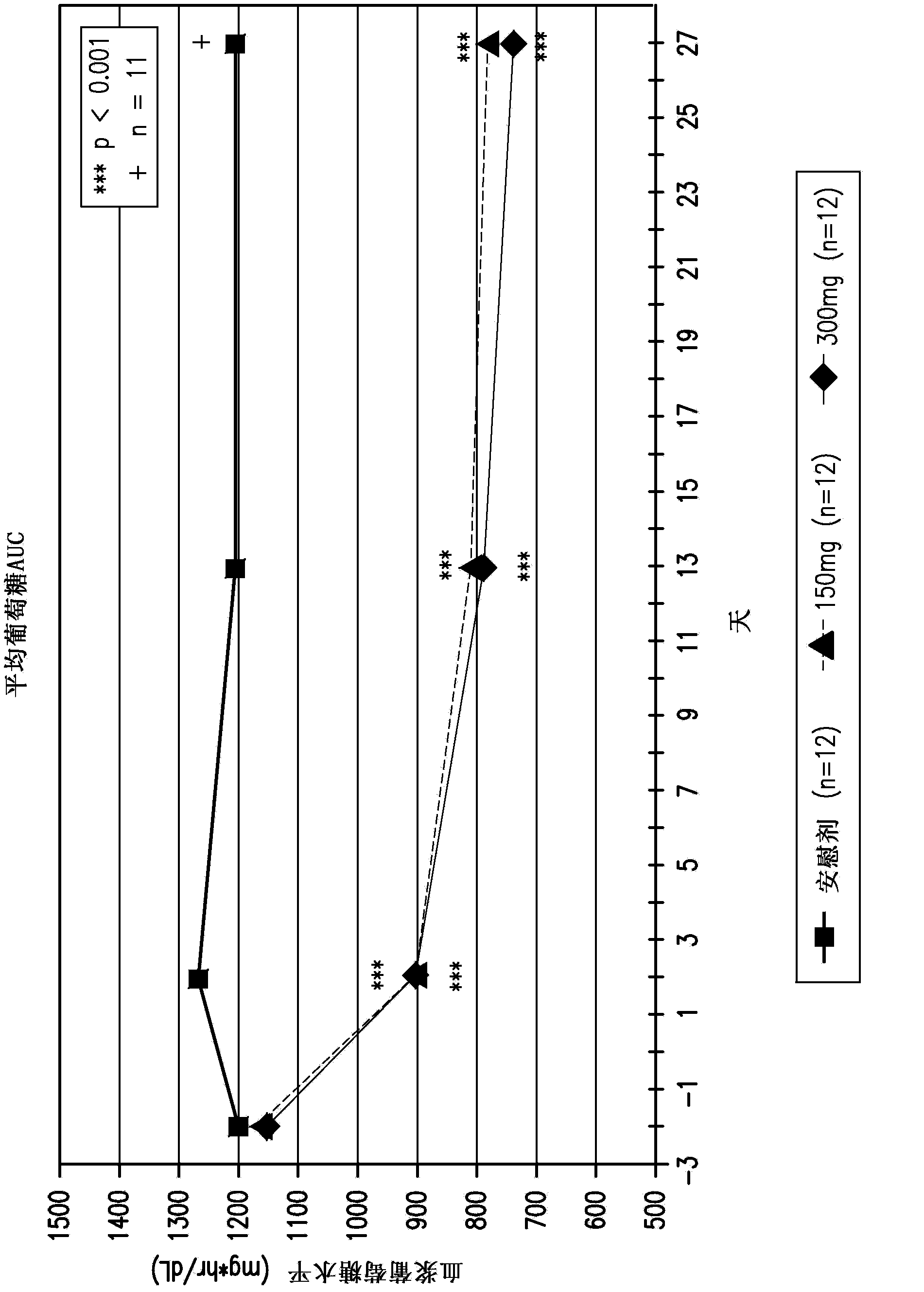
Compositions comprising inhibitors of sodium-glucose cotransporters 1 and 2 and using methods
A dosage form and therapeutic agent technology, applied in the field of compounds and pharmaceutical compositions, can solve problems such as glucose-galactose malabsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0264] In vitro human SGLT2 inhibition assay
[0265] Human sodium / glucose cotransporter type 2 (SGLT2; accession number P31639; GI:400337) was cloned into the pIRESpuro2 vector for mammalian expression (construct: HA-SGLT2-pIRESpuro2).
[0266] HEK293 cells were transfected with the human HA-SGLT2-pIRESpuro2 vector, and a large number of stable cell lines were selected in the presence of 0.5 μg / ml puromycin. Human HA-SGLT2 cells were maintained in DMEM medium containing 10% FBS, 1% GPS and 0.5 μg / ml puromycin.
[0267] HEK293 cells expressing human HA-SGLT2 were seeded in 384-well plates (30,000 cells / well) in DMEM medium containing 10% FBS, 1% GPS, and 0.5 μg / ml puromycin, and then incubated at 37 °C, 5%CO 2 Incubate overnight. Then with uptake buffer (140mM NaCl, 2mM KCl, 1mM CaCl 2 , 1 mM MgCl 2 , 10mM HEPES, 5mM Tris, 1mg / ml bovine serum albumin (BSA), pH7.3) to wash the cells. 20 microliters of uptake buffer with or without test compound was added to the cells. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com