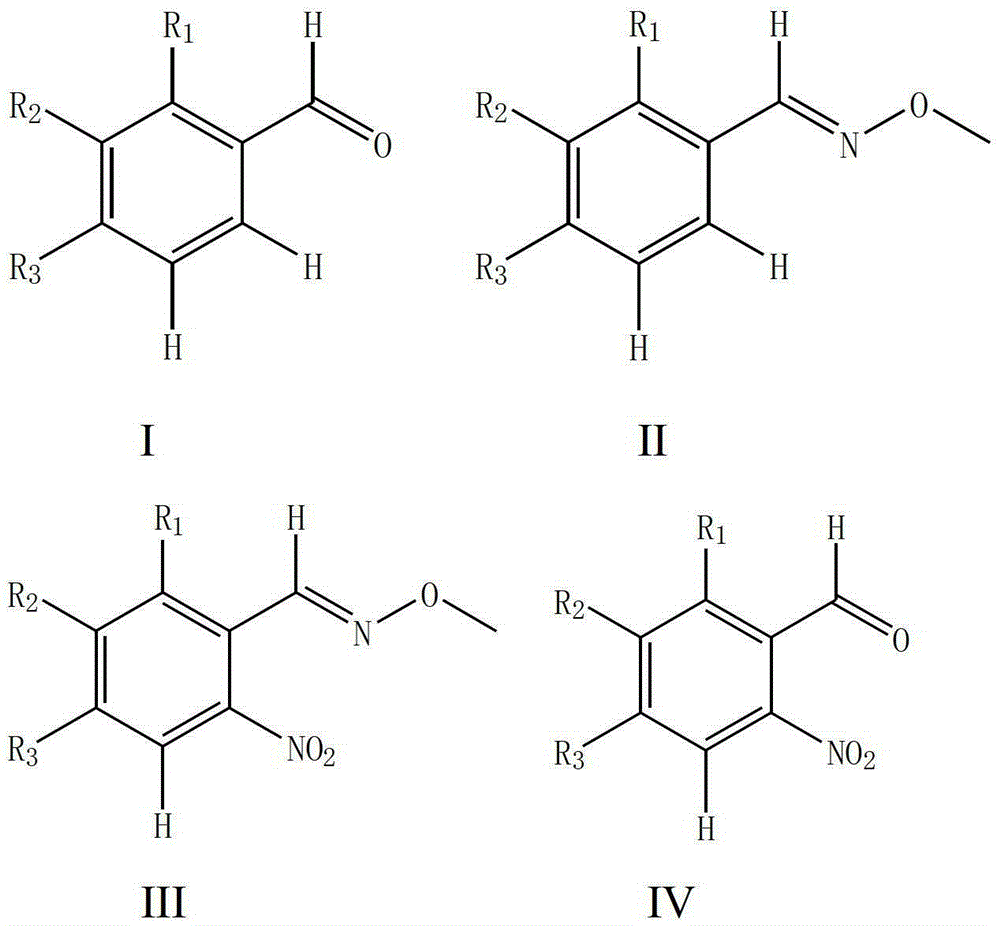
A kind of synthetic method of o-nitrobenzaldehyde compound
An o-nitrobenzaldehyde and compound technology, which is applied in the field of organic compound synthesis, can solve problems such as different reaction steps, long reaction steps, strong acid safety issues, etc., and achieves good selectivity, simple reaction steps, and good substrate adaptability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
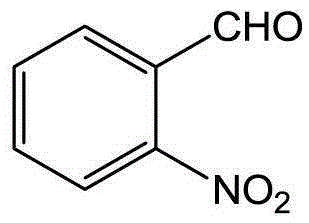
[0045] Using benzaldehyde as raw material to synthesize 2-nitrobenzaldehyde
[0046]
[0047] (1) Add 0.530g (5.0mmol) of benzaldehyde, 1.12g (13.4mmol) of methoxyamine hydrochloride, 1.804g (22mmol) of anhydrous sodium acetate, 15ml of ethanol, and 45ml of water into a 100ml flask. The mixture was heated to reflux reaction, followed by TLC detection, the reaction was completed after 3 hours, the resulting mixed solution was extracted with 3×45mL ethyl acetate, the organic phase was taken and dried, and the solvent was removed under reduced pressure to obtain a light yellow liquid benzaldehyde-O-methyl Oxime 0.641g (95% yield).
[0048](2) Add 67.5 mg (0.5 mmol) of benzaldehyde O-methyloxime, 11 mg (0.05 mmol) of palladium diacetate, 154 mg (1.0 mmol) of silver nitrite, 270 mg (1.0 mmol) of potassium persulfate and 1,2-bis Ethyl chloride (5ml) was successively added to a 10ml sealed pressure vessel. The mixture was heated and reacted in an oil bath at 110°C, followed by T...
Embodiment 2
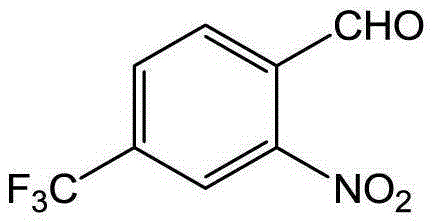
[0052] Synthesis of 4-trifluoromethyl-2-nitrobenzaldehyde from 4-trifluoromethylbenzaldehyde
[0053]
[0054] (1) Add 0.870g (5.0mmol) of 4-trifluoromethylbenzaldehyde, 1.12g of methoxyamine hydrochloride, 1.804g of anhydrous sodium acetate, 15ml of ethanol, and 45ml of water into a 100ml flask. The mixture was heated to reflux reaction, followed by TLC detection, and the reaction was completed after 3 hours. The resulting mixed solution was extracted with 3 × 45mL ethyl acetate, the organic phase was taken and dried, and the solvent was removed under reduced pressure to obtain a light yellow liquid 4-trifluoromethylbenzene Formaldehyde-O-methyloxime 0.995g (98% yield).
[0055] (2) 101.5mg (0.5mmol) of 4-trifluoromethylbenzaldehyde-O-methyloxime, 11mg (0.05mmol) of palladium diacetate, 154mg (1.0mmol) of silver nitrite, 270mg (1.0mmol) of potassium persulfate ) and 1,2-dichloroethane (5ml) were sequentially added to a 10ml sealed pressure vessel. The mixture was heated ...
Embodiment 3
[0059] Synthesis of 5-bromo-2-nitrobenzaldehyde from 3-bromobenzaldehyde
[0060]
[0061] (1) Add 0.915g (5.0mmol) of 3-bromobenzaldehyde, 1.12g of methoxyamine hydrochloride, 1.804g of anhydrous sodium acetate, 15ml of ethanol, and 45ml of water into a 100ml flask. The mixture was heated to reflux reaction, followed by TLC detection, the reaction was completed after 3 hours, the resulting mixed solution was extracted with 3 × 45mL ethyl acetate, the organic phase was taken and dried, and the solvent was removed under reduced pressure to obtain a light yellow solid 3-bromobenzaldehyde-O - Methyloxime 0.994 g (98% yield).
[0062] (2) 101.5mg (0.5mmol) of 3-bromobenzaldehyde-O-methyloxime, 11mg (0.05mmol) of palladium diacetate, 154mg (1.0mmol) of silver nitrite, 270mg (1.0mmol) of potassium persulfate and 1 , 2-dichloroethane (5ml) was sequentially added to a 10ml sealed pressure vessel. The mixture was heated and reacted in an oil bath at 110°C, followed by TLC detectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com