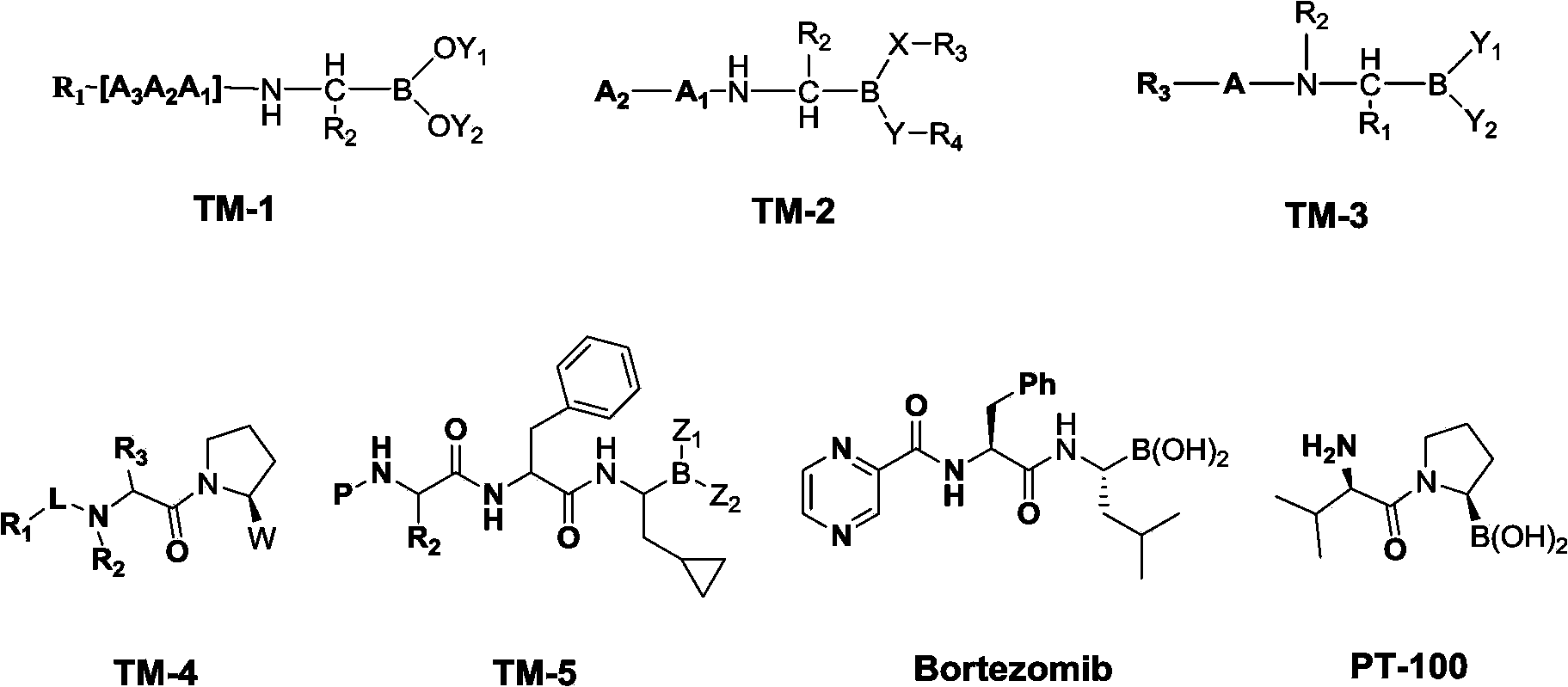
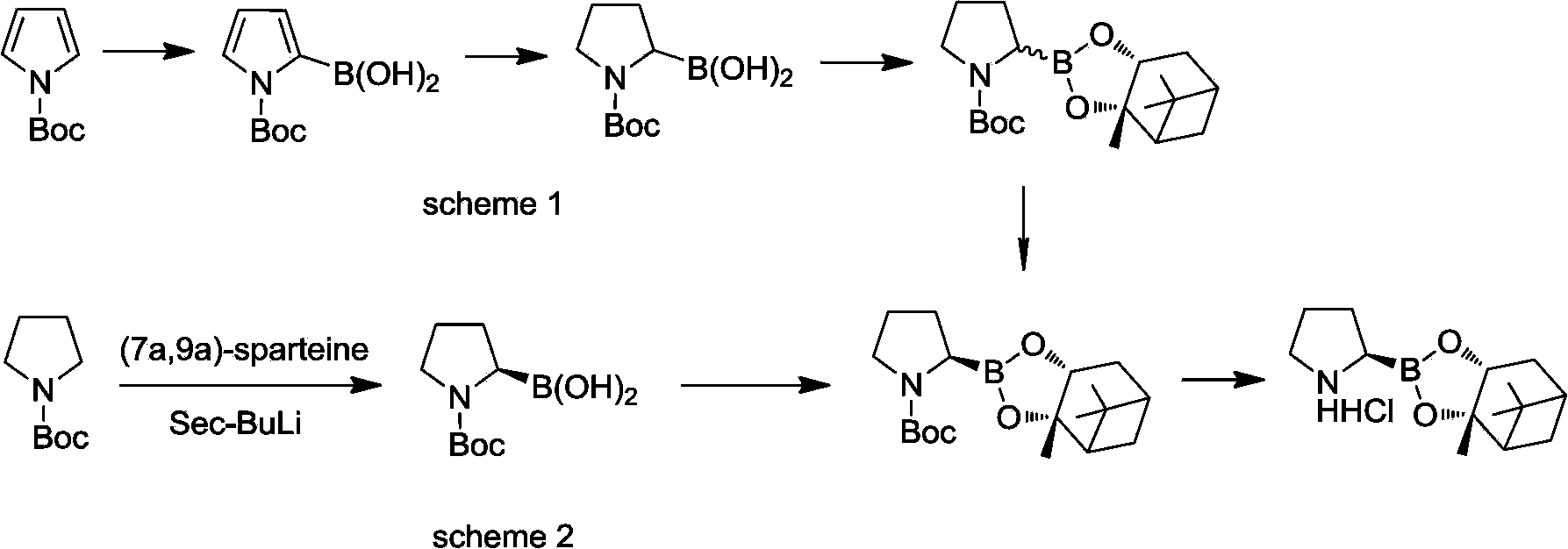
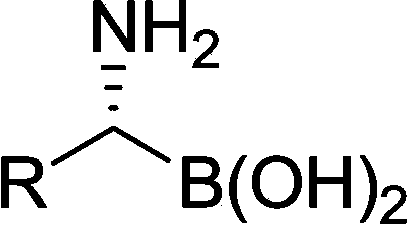Diverse chiral amino boric acid, preparation method and application thereof
An aminoboronic acid, a variety of technologies, applied in chemical instruments and methods, active ingredients of boron compounds, compounds containing elements of Group 3/13 of the periodic table, etc. Problems such as poor yield of aminoboronic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Preparation and Characterization of Aminoboronate:
[0146]
[0147] Compound(1). Use general method 1 to react.
[0148] Add 295 mg (2 mmol) of tert-butylsulfinimide Compound 1' and react for 48 hours.
[0149] The reaction mixture was purified by column chromatography on silica gel deactivated with water, and the developing solvent was chloroform / methanol system. The obtained product was a pale yellow oil (253 mg, yield: 85%) at room temperature.
[0150] 1 H-NMR(DMSO,δ,ppm):4.78(d,j=6.4,0.43H,NH),4.34(dd,j 1 =1.6Hz,j 2 =8.8Hz,1H),2.93(m,1H,CHN),2.30(m,1H),2.17(m,1H),1.97(m,1H),1.87(m,1H),1.73(m,1H) ,1.32(s,3H),1.26(s,3H),1.20(d,j=7.2Hz,3H),1.12(m,1H),1.09(s,9H),0.82(s,3H). 13 C-NMR (DMSO, δ, ppm): 85.9, 77.5, 55.5, 51.3, 38.3, 35.4, 28.8, 27.3, 26.3, 24.1, 23.1, 19.2, 19.1.
Embodiment 2
[0152]
[0153] The reaction was carried out using General Method 2.
[0154] Add 351 mg (2 mmol) of tert-butylsulfinimide Compound 2' and react for 48 hours.
[0155] The reaction mixture was purified by column chromatography on silica gel deactivated with water, and the developing solvent was chloroform / methanol system. The obtained product was a pale yellow oil (322 mg, yield: 92%) at room temperature.
[0156] 1 H-NMR(DMSO,δ,ppm):4.76(d,j=6.8Hz,1H,NH),4.34(dd,j 1 =2Hz,j 2 =8.8Hz,1H),2.82(m,1H,CHN),2.30(m,1H),2.16(m,1H),1.97(m,1H),1.87(m,1H),1.71(m,1H) ,1.56(m,2H),1.34(m,2H),1.32(s,3H),1.25(s,3H),1.13(m,1H),1.08(s,9H),0.87(t,j=7.2 Hz,3H),0.82(s,3H). 13 C-NMR (DMSO, δ, ppm): 85.9, 77.4, 55.7, 51.2, 38.3, 35.5, 35.5, 28.8, 27.3, 26.4, 24.1, 23.1, 20.1, 14.5.
Embodiment 3
[0158]
[0159] The reaction was carried out using General Method 1.
[0160] Add 351 mg (2 mmol) of imine Compound 3' and react for 48 hours. The reaction mixture was purified by column chromatography on silica gel deactivated with water, and the developing solvent was chloroform / methanol system. The obtained product was a pale yellow oil (308 mg, yield: 88%) at room temperature.
[0161] 1 H-NMR(DMSO,δ,ppm):4.73(d,j=6.8Hz,1H,NH),4.34(m,1H),2.66(t,j=6.4Hz,1H,CHN),2.32(m, 1H),2.16(m,1H),1.98(m,1H),1.87(m,2H),1.70(m,1H),1.32(s,3H),1.25(s,3H),1.18(m,1H ),1.09(s,9H),0.92(d,j=7.2Hz,6H),0.82(s,3H). 13C-NMR (DMSO, δ, ppm): 77.4, 55.9, 51.2, 38.3, 35.5, 31.2, 28.9, 27.3, 26.5, 24.2, 23.0, 20.8, 20.0.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap