Preparation method of porous morphology high voltage lithium nickel manganese oxide cathode material
A technology of lithium nickel manganese oxide and positive electrode materials, which is applied in the field of preparation of lithium nickel manganese oxide positive electrode materials with porous morphology and high voltage, can solve the problems of complex preparation process, capacity attenuation, volume change, etc., and achieve simple synthesis process and increased magnification Performance, the effect of changing the specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0022] Specific embodiment one: this embodiment prepares lithium nickel manganese oxide material according to the following steps:
[0023] Weigh 0.03mol of manganese carbonate and calcinate in a muffle furnace at 800°C for 4 hours to obtain porous manganese trioxide; mix the above manganese trioxide with 0.01mol of nickel acetate and 0.02mol of lithium nitrate by ball milling for 2 hours to obtain a precursor; Put the above precursor in a muffle furnace, pre-calcine at 500°C for 4 hours, and calcinate at 850°C for 10 hours to obtain a porous lithium nickel manganese oxide material. The SEM image is as follows figure 1 shown.
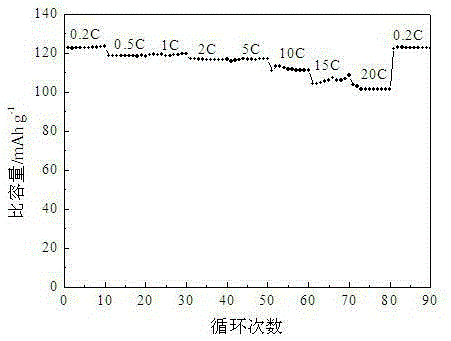
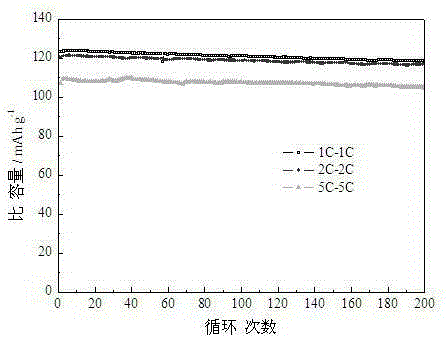
[0024] The pore volume of lithium nickel manganese oxide material prepared in this embodiment is 0.3 cm 3 / g, the specific surface area is 13m 2 / g. Such as Figure 2-3 As shown, the specific capacity can reach 101.3mAh / g when discharged at 20C, and the specific capacity is 118.2mAh / g, 116.7mAh / g and 104.6mAh / g after 200 charge-discharge cycles at 1...
specific Embodiment approach 2
[0025] Specific embodiment two: this embodiment prepares lithium nickel manganese oxide material according to the following steps:
[0026] Weigh 0.03mol of manganese carbonate and calcinate in a muffle furnace at 450°C for 4 hours to obtain porous manganese dioxide; disperse the above manganese dioxide, 0.01mol of nickel nitrate, 0.01mol of lithium acetate and 0.012mol of lithium hydroxide into 20ml of ethanol , stirring and volatilizing ethanol at room temperature to obtain a precursor; placing the above precursor in a muffle furnace, pre-calcining at 400°C for 3h, and calcining at 800°C for 12h to obtain a porous lithium nickel manganese oxide material.
[0027] The pore volume of lithium nickel manganese oxide material prepared in this embodiment is 0.25m 3 / g, the specific surface area is 22m 2 / g. When discharged at 10C, the specific capacity can reach 111.3mAh / g, and after 200 charge-discharge cycles at 2C rate, the specific capacity is 117.9mAh / g, and the capacity re...
specific Embodiment approach 3
[0028] Specific embodiment three: this embodiment prepares lithium nickel manganese oxide material according to the following steps:
[0029] Weigh 0.03mol of manganese oxalate and calcinate in a muffle furnace at 280°C for 6h to obtain porous manganese dioxide; disperse the above manganese dioxide, 0.005mol of nickel nitrate, 0.005mol of nickel formate, and 0.02mol of lithium formate into 15ml of ethanol , stirring and volatilizing ethanol at room temperature to obtain a precursor; placing the above precursor in a muffle furnace, pre-calcining at 400°C for 4 hours, and calcining at 800°C for 12 hours to obtain a porous lithium nickel manganese oxide material.
[0030] The pore volume of lithium nickel manganese oxide material prepared in this embodiment is 0.3m 3 / g, the specific surface area is 18m 2 / g. The specific capacity can reach 114.3mAh / g when discharged at 10C, and the specific capacity is 121.0mAh / g after 200 charge-discharge cycles at 2C rate, and the capacity r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com