Tamoxifen citrate freeze-dried powder injection
A technology of tamoxifen and freeze-dried powder injection, applied in the field of tamoxifen citrate freeze-dried powder injection, can solve the problems of high cost, low bioavailability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
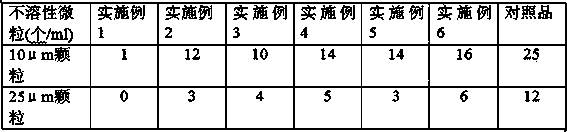
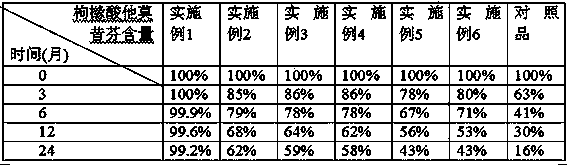
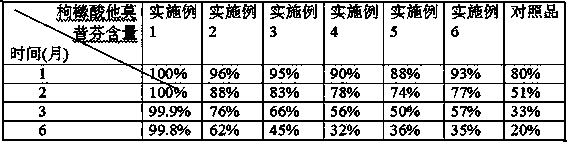
Examples
Embodiment 1
[0026] Tamoxifen Citrate 86g
[0027] The composition of sorbitol, dextran and lactose (the weight ratio of the three is 3.2:5.5:2) 233g
[0028] The composition of arginine and glutathione (the weight ratio of the two is 1.6:5.7) 14.5g
[0029] Disodium edetate 10g
[0030] Hydrochloric acid solution (0.1mol / l) appropriate amount to adjust the pH to 6.5
[0031] Add water for injection to 1000ml
[0032] Preparation:
[0033] Weigh the prescribed amount of tamoxifen citrate, sorbitol, dextran, lactose, arginine, glutathione and disodium edetate respectively, add to water for injection, stir well to dissolve, add activated carbon , adsorption, decarburization and filtration. The hydrochloric acid solution adjusted the pH to 6.5. After filtering with a 0.22μm filter membrane, fill it into washed, dried and sterilized vials, each containing 3mL, half-press the cap, turn on the freeze dryer, cool down to -40°C, freeze-dry, crimp the cap, and check ,Package.
Embodiment 2
[0035] Tamoxifen Citrate 86g
[0036] The composition of mannitol, glucose and dextran (the weight ratio of the three is 3:5:2) 233g
[0037] The composition of arginine and glutathione (the weight ratio of the two is 1.6:5.7) 14.5g
[0038] Disodium edetate 10g
[0039] Hydrochloric acid solution (0.1mol / l) appropriate amount to adjust the pH to 6.5
[0040] Add water for injection to 1000ml
[0041] Preparation method is the same as embodiment 1
Embodiment 3
[0043] Tamoxifen Citrate 86g
[0044] The composition of sorbitol, dextran and lactose (the weight ratio of the three is 3.2:5.5:2) 233g
[0045] The composition of cysteine hydrochloride and glutathione (the weight ratio of the two is 1:5) 14.5g
[0046] Disodium edetate 10g
[0047] Hydrochloric acid solution (0.1mol / l) appropriate amount to adjust the pH to 6.5
[0048] Add water for injection to 1000ml
[0049] Preparation method is the same as embodiment 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com