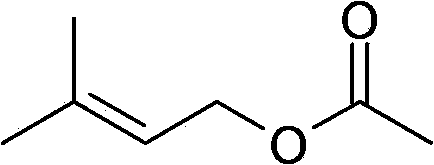
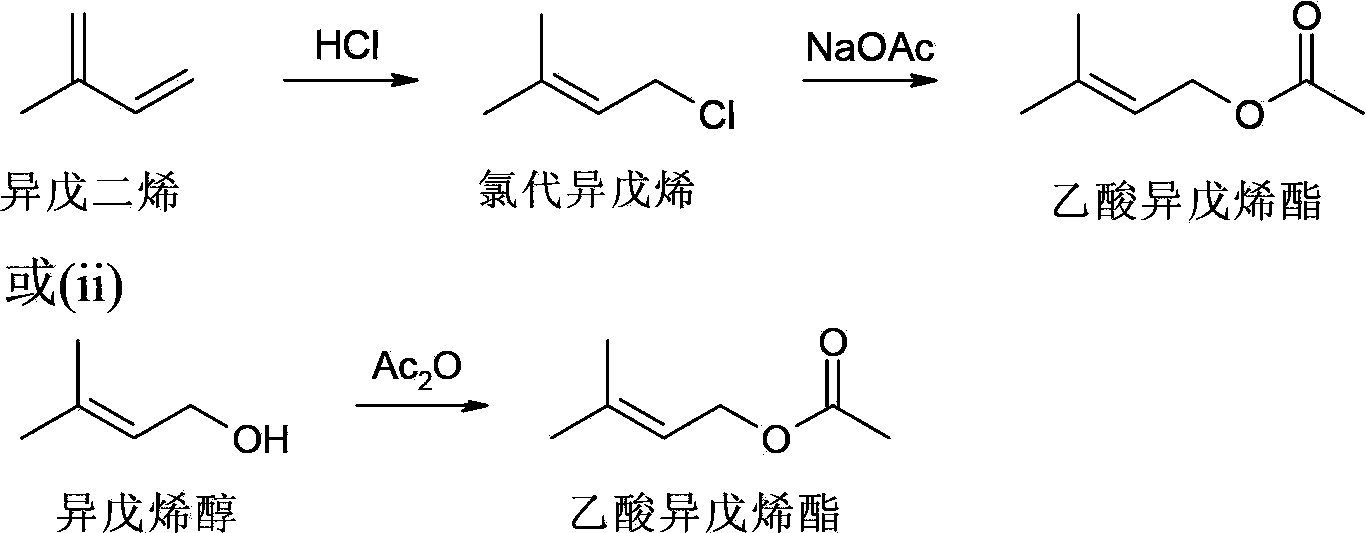
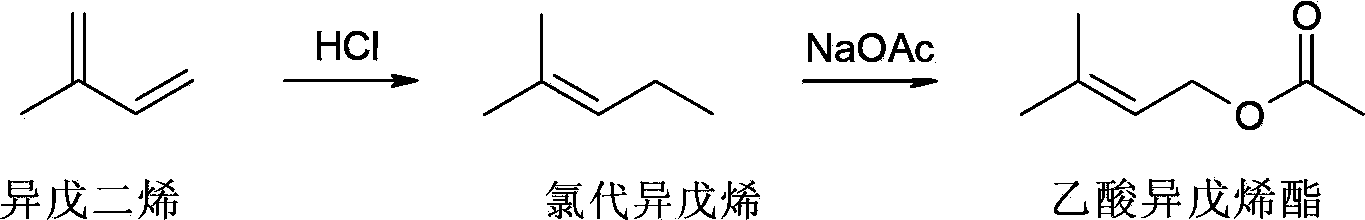
Isopentene acetate and preparation method thereof
A technology of isopentenyl acetate and isoprene, which is applied in the field of isopentenyl acetate and its preparation, and can solve problems such as the inability to predict the commercial scale of fragrance molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0015]
[0016] Preparation of isopentenyl acetate: Into a three necked round bottom flask equipped with a stirrer and dropping funnel was added isoprene (2.54Kg). The temperature of the flask was cooled to 0 °C. Sodium chloride (NaCl) was divided into 8 equal portions (100 g each). A first portion of sodium chloride (100 g) was added to the flask, followed by concentrated HCl (1.1 Kg) over 0.5 hours. Additional portions of sodium chloride were then added approximately every 15 minutes along with conc. HCl. The total amount of concentrated HCl added was about 12 Kg. The reaction was aged for about 2 hours. The organic layer (1.96Kg) was transferred to a solution containing solid sodium carbonate (Na 2 CO 3 ) (150g) in a separatory funnel to obtain isopentyl chloride. In the presence of triethylamine (Et3N), chloroisoamylene is further reacted with sodium acetate (NaOAc) at 60 ° C to obtain the product prenyl acetate, which has a boiling point of 60 at a pressure of 10...
Embodiment II
[0019]
[0020] Preparation of isopentenyl acetate: Acetic anhydride ((CH 3 COO) 2 O, Ac 2 O) (516g) and anhydrous sodium acetate (CH 3 COONa) (5 g). The temperature of the flask was heated to 100°C. Prenol (408 g) was then added slowly over 2 hours while maintaining the temperature at 105-110°C. The reaction was aged for at least 1.5 hours to achieve maximum conversion. The reaction was cooled to 80 °C. Dispose of excess acetic anhydride with water. The organic layer was extracted and washed with aqueous sodium carbonate to give crude prenyl acetate (590 g). Further distillation gave the product isopentenyl acetate, which boiled at 60°C at a pressure of 10 mmHg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com