Soluble human M-CSF receptor and uses thereof
A soluble, receptor-specific technology for diagnosing disease and symptoms to address rising morbidity and mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
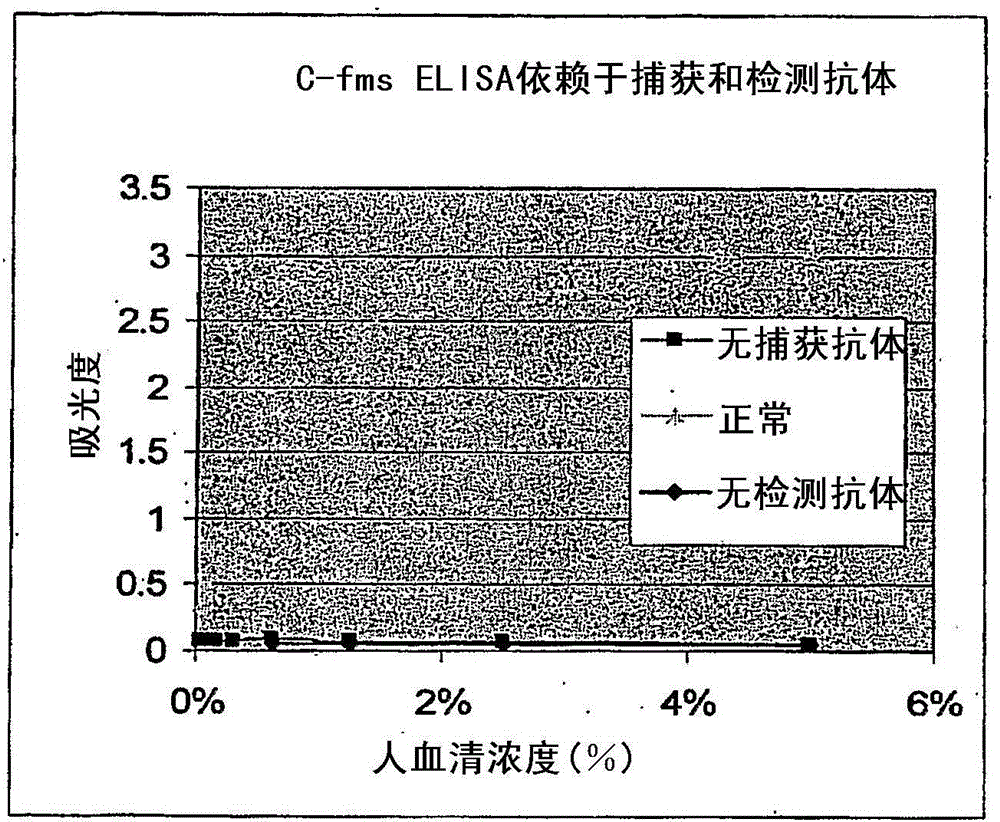
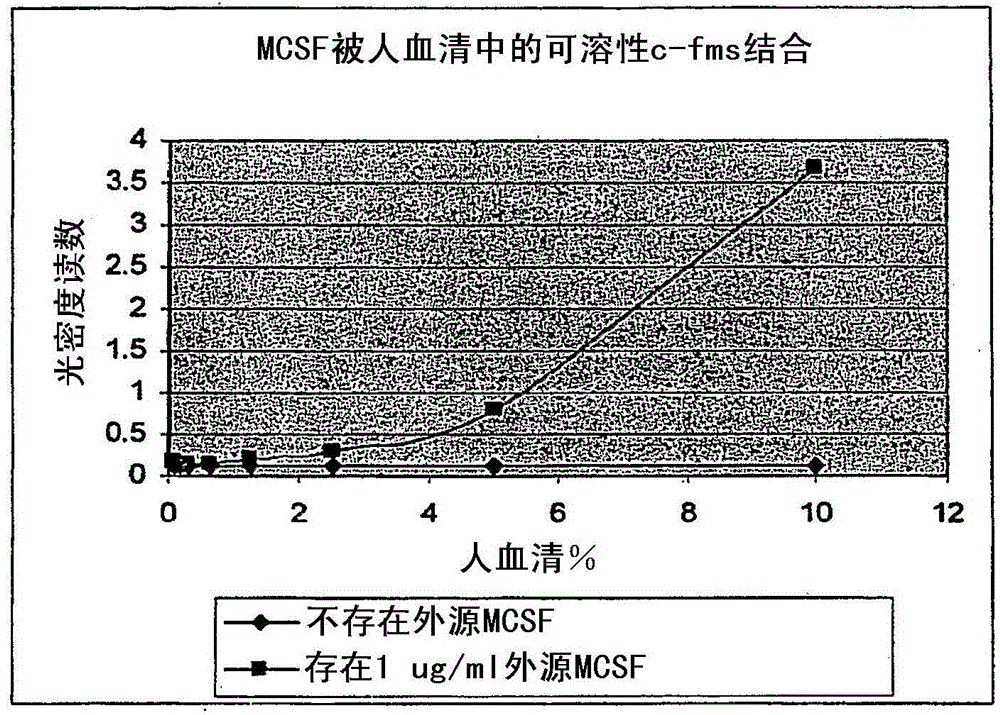
[0096] This example describes the identification of a soluble receptor for human M-CSF and shows that the soluble receptor is capable of binding M-CSF.
[0097]I. Identification of Soluble Human M-CSF Receptor in Serum Samples
[0098] A. Materials and methods
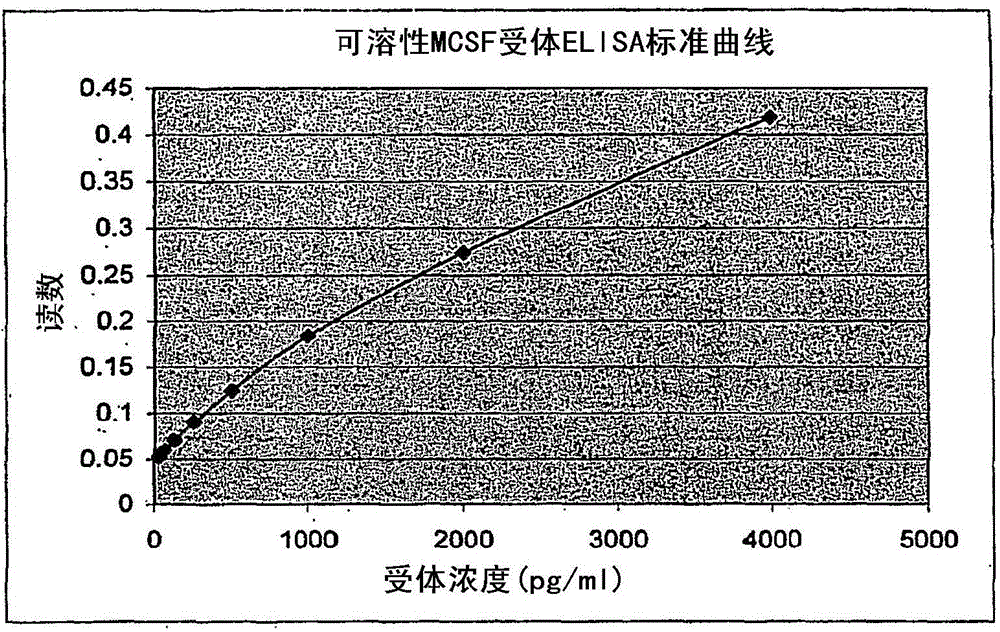
[0099] A 100 ng / ml capture antibody (DuoSet Elisa Development System hM-CSF R (R&D Systems, catalog number DY329)) working solution prepared in PBS was used to coat a microtiter plate (R&D Systems, catalog number CP0011) with 100 μl / well. Plates were sealed and incubated overnight at room temperature. After washing each well 3 times with washing buffer (0.05% Tween in PBS, pH7.2-7.4) using a multi-channel dispenser / washer, the plate was washed with 300 μl / well blocking buffer (1% BSA, 5% Sucrose, pH 7.2-7.4) for at least 1 hour at room temperature.
[0100] After repeated washing steps, samples can be added to the plate. Samples or standards diluted with diluent were added at 100 μl / well. Plates were covered with ...
Embodiment 2
[0119] This example shows that soluble M-CSF receptors are found in human urine samples. This example also shows that this receptor is present in the serum of normal subjects and breast cancer patients and that the level of soluble M-CSF receptor correlates with the level of M-CSF. Finally, this example shows that soluble M-CSF receptors are also found in primates.
[0120] I. Soluble human M-CSF receptor was also found in urine samples
[0121] Urine samples collected from healthy human volunteers were assayed for the presence of soluble human M-CSF receptor using the same ELISA setup as described in Example 1. Different levels of soluble human M-CSF receptor were detected in adult females (25-66 years), adult males (35-70 years) and children 3-9 years old. The recombinant c-fms-human Fc fusion protein was used as a reference to quantify the concentration of soluble human M-CSF receptor ( Figure 9 ).
[0122] Urine samples were collected daily throughout the menstrual cy...
Embodiment 3
[0130] This example shows that the expression of soluble human M-CSF receptor is closely related to osteoclast differentiation. This example also shows that only differentiated osteoclasts express the soluble human M-CSF receptor, the level of which increases with M-CSF removal.
[0131] A. Materials and methods
[0132] Expression of the M-CSF soluble receptor was studied in an in vitro system using human osteoclasts, in which expression of the membrane-bound M-CSF receptor was found. Specifically, the expression of soluble receptors was detected during osteoclast differentiation. Primary human osteoclast precursors (Cambrex Bio Science Walkersville, Inc., Cat. No. 2T-110) were seeded at 10,000 cells / well and 0.2ml / well in 30ng / ml human Cell culture medium with M-CSF and 100 ng / ml RANKL. Add 1 μg / mL detection antibody to each well on the same day as cell seeding. At 37°C, 5% CO 2 Cells were grown in humid air. Osteoclasts were identified by phase contrast microscopy on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com