Synthetic method for stable isotope labeled N,N-dimethylaminochloropropane hydrochloride
A dimethylaminochloropropane, isotope labeling technology, applied in the preparation of amino compounds, chemical instruments and methods, preparation of amino-substituted functional groups, etc. Inapplicability and other problems, to achieve the effects of good economy and practical application value, simple post-processing purification process, and high atom utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
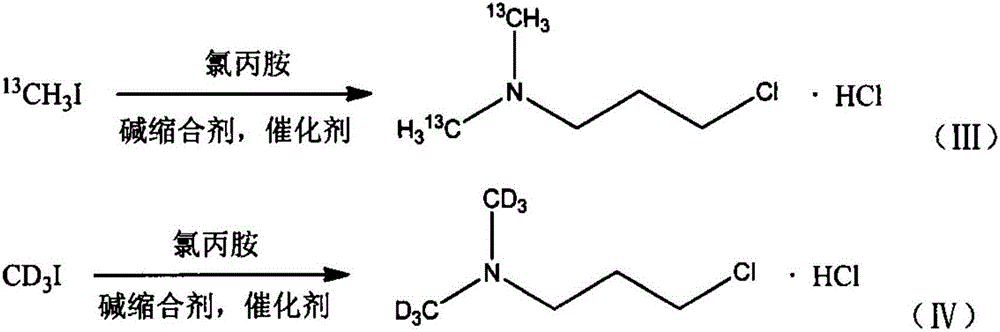
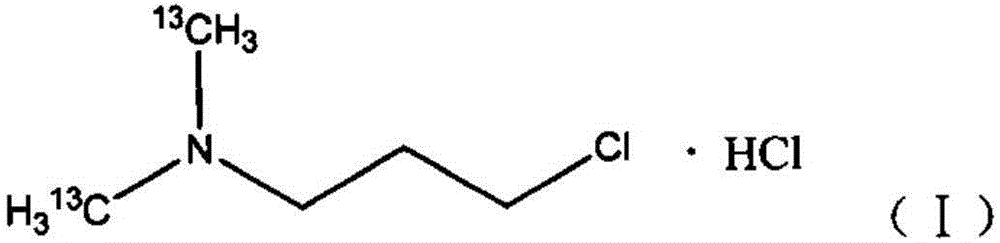
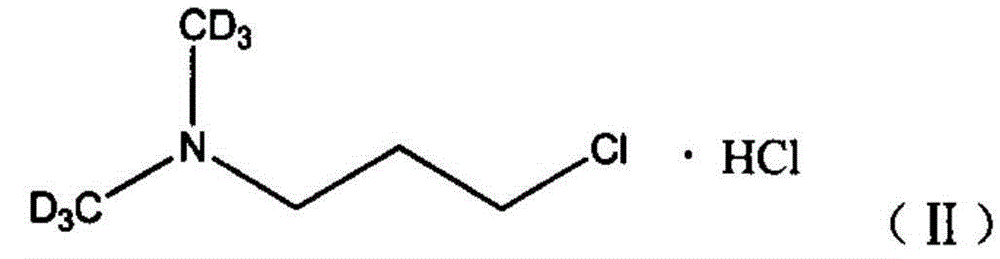
[0034] a stable isotope 13 C mark N, the synthetic method of N-dimethylaminochloropropane hydrochloride, the specific synthetic process of this method comprises the following steps:
[0035] In a three-neck flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, the isotope 13 The methyl iodide of C mark and chlorpromamine hydrochloride are added in the there-necked flask in molar ratio 1:1, then add the catalyst tetrabutylammonium bromide of 1mol% (calculated as chlorpromamine hydrochloride) and concentration be 25wt% Aqueous sodium hydroxide solution, wherein the molar ratio of sodium hydroxide to chlorpropylamine hydrochloride is 5:1, the solvent is water, react at normal pressure and 35°C for 7 hours; after the reaction is completed, cool to room temperature, and the reaction liquid is separated, After purification, N,N-dimethylaminochloropropane is obtained; dry hydrogen chloride gas is passed into N,N-dimethylaminochloropropane, and stable isotope-...
Embodiment 2
[0037] a stable isotope 13 C mark N, the synthetic method of N-dimethylaminochloropropane hydrochloride, the specific synthetic process of this method comprises the following steps:
[0038] In a three-neck flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, the isotope13 The methyl iodide of C mark and chlorpromamine hydrochloride are added in the there-necked flask by molar ratio 2:1, then add the catalyst benzyltriethylammonium chloride of 1mol% (calculated as chlorpromamine hydrochloride) and concentration is 10wt % sodium hydroxide aqueous solution, wherein the molar ratio of sodium hydroxide to chlorpromamine hydrochloride is 3:1, the solvent is water, and react at normal pressure and 65°C for 12h; after the reaction is completed, cool to room temperature, and the reaction solution is After separation and purification, N,N-dimethylaminochloropropane is obtained; dry hydrogen chloride gas is passed into N,N-dimethylaminochloropropane, and stable ...
Embodiment 3
[0040] a stable isotope 13 C mark N, the synthetic method of N-dimethylaminochloropropane hydrochloride, the specific synthetic process of this method comprises the following steps:
[0041] In a three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, the isotope 13 The iodomethane and chlorpromamine hydrochloride of C mark join in the there-necked flask by molar ratio 4:1, then add the catalyst benzyltriethylammonium chloride of 0.5mol% (calculated as chlorpropylamine hydrochloride) and concentration is 50wt% sodium carbonate toluene solution, wherein the molar ratio of sodium carbonate to chlorpropylamine hydrochloride is 8:1, the solvent is toluene, react at normal pressure and 95°C for 7h; after the reaction is completed, cool to room temperature, and the reaction solution is After separation and purification, N,N-dimethylaminochloropropane is obtained; dry hydrogen chloride gas is passed into N,N-dimethylaminochloropropane, and stable i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com