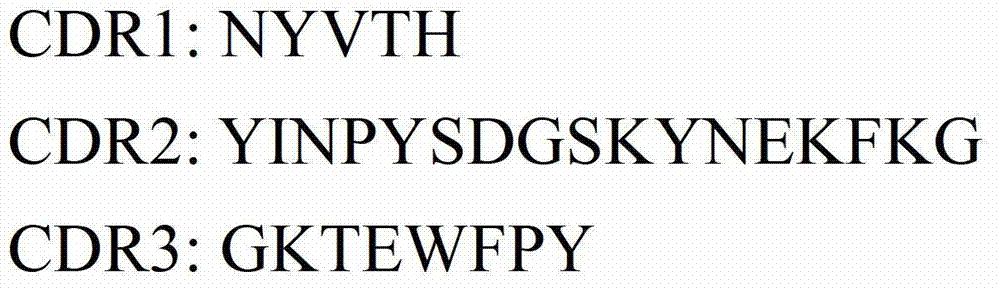Anti-idiotype antibody for human CD22 antibody, and application thereof
An antibody and antibody heavy chain technology, applied in applications, specific peptides, recombinant DNA technology, etc., can solve problems such as difficulty in biological activity detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Example 1. Anti-idiotypic antibodies against human CD22 antibodies in the form of single-chain antibodies
[0113] In this example, the variable region light and heavy chain sequences of the anti-SM03 anti-idiotypic antibody were obtained by using phage display library technology, and the anti-idiotypic single-chain antibody against human CD22 antibody—the soluble scFv expressed by phage #3 was further prepared. Specific steps are as follows:
[0114] 1. Preparation of phage display library by injecting SM03 immunized mice
[0115] About 6 weeks old female Balb / c mice were immunized by intraperitoneal injection of 100ug SM03 monoclonal antibody according to the standard immunization procedure (see "In Antibodies: A Laboratory Manual" published by Cold Spring Harbor Laboratory, USA in 1988), And emulsified with 200uL complete Freund's adjuvant (product of Sigma-Aldrich, USA). The second and third immunizations were carried out after 14 days and 35 days respectively. Th...
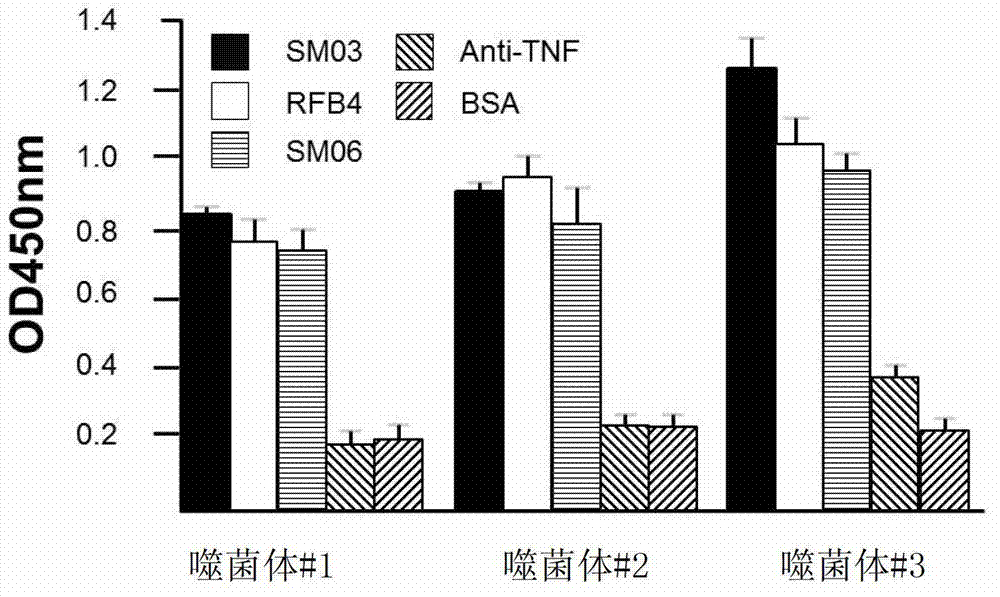
experiment example 2
[0124] Experimental example 2, using the soluble scFv expressed by phage #3 in Example 1 to conduct pharmacokinetic research in SM03 clinical trials
[0125] Due to the specificity of the compatible scFv expressed by phage #3 in Example 1 to SM03, it can be used to develop a corresponding detection method to determine the antibody concentration in the serum of patients receiving anti-CD22 antibody treatment, especially to assist clinical practice. required pharmacokinetic studies. A 96-well ELISA plate was coated with the compatible scFv expressed by phage #3 in Example 1. After blocking and washing with BSA, the serum of each blood collection point of patients receiving SM03 treatment was diluted and added to the wells. After incubation at 37°C for 2 hours and thorough washing, HRP-labeled goat anti-human Fc antibody (product of Jackson Immunoresearch) was diluted 1:4000 and added to the wells. After incubation at 37°C for 1 hour, the microtiter plate was washed 5 times, de...
Embodiment 3
[0127] Example 3. Anti-idiotypic antibody IdmG2a / k for human CD22 antibody in the form of murine IgG2a / kappa immunoglobulin molecules
[0128] The preparation of compatible scFv expressed by phage #3 in Example 1 requires a series of complex steps such as bacterial culture, induced expression and collection of inclusion bodies, protein denaturation and folding, and His-tag purification. Furthermore, scFvs are less stable and difficult to store. In order to produce a molecule that not only retains the adsorption specificity of the soluble scFv expressed by phage #3 in Example 1, but also can be purified by standard procedures and is stable and easy to store, the variable region of the heavy chain of the soluble scFv expressed by phage #3 in Example 1 (VH) coding DNA and light chain variable region (VK) coding sequences were amplified by PCR and integrated into the amplifiable expression vectors pIdmkappa-VK and pIdmIgG-VH using molecular cloning techniques, which also contained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com