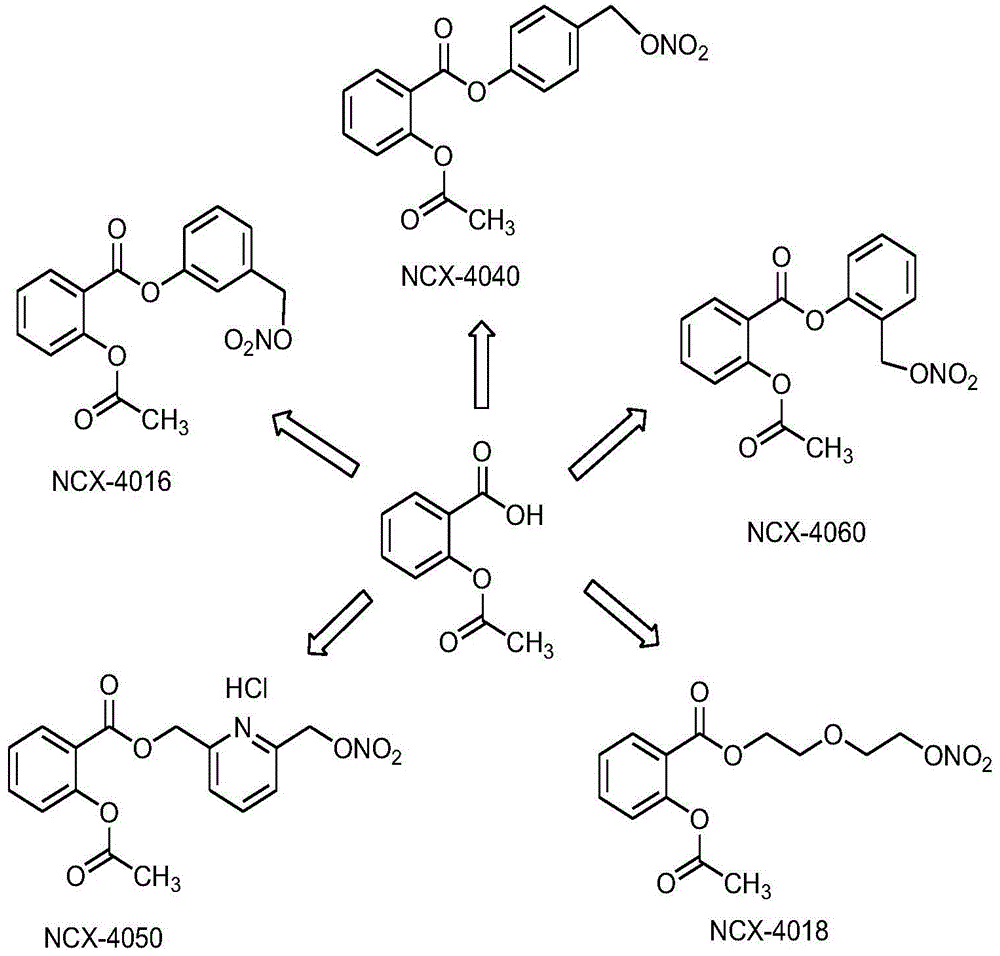
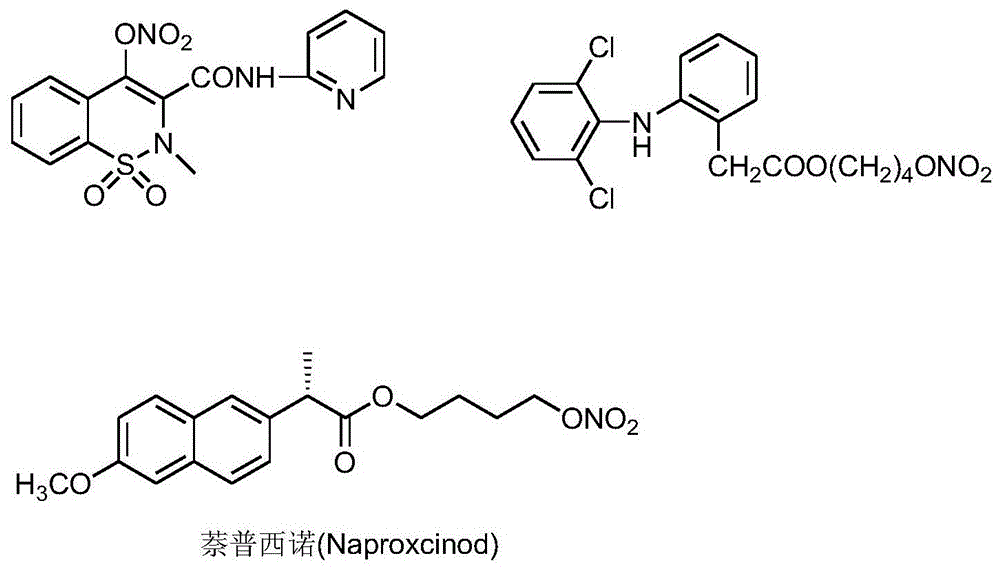
Nitric oxide-donating non-steroidal anti-inflammatory drug β-cyclodextrin or its derivative inclusion compound and its preparation method and use
A non-steroidal anti-inflammatory drug, nitric oxide technology, applied in the field of medicine, can solve the problems of easy sticking, inconvenient use, excessive mass or volume, etc., and achieves the effect of excellent water solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1 Preparation of Naproxino-sulfobutyl ether-β-cyclodextrin inclusion compound
[0110] Weigh 4kg of clathrate material sulfobutyl ether-β-cyclodextrin, add 10L of distilled water and stir until completely dissolved; add 350g of naproxil into 2L of ethyl acetate, stir and dissolve completely; mix the above two solutions and add tetrabutyl Ammonium bromide 1g, after stirring vigorously for 60min, let it stand, and separate the liquids; reclaim the ethyl acetate layer, and concentrate the ethyl acetate solution to dryness under reduced pressure to reclaim the unincluded bulk drug; the separated aqueous solution is washed with ethyl acetate (0.5L×3) and then spray-dried to obtain 3.88kg of loose naproxino-sulfobutyl ether-β-cyclodextrin inclusion compound. The inclusion rate was detected by HPLC method to be 76%. 46.4 g of naproxino was recovered.
Embodiment 2
[0111] Example 2 Preparation of NCX-4060-sulfobutyl ether-β-cyclodextrin inclusion compound
[0112] Weigh 180g of inclusion material sulfobutyl ether-β-cyclodextrin, add 200ml of distilled water and stir until completely dissolved, add 15g of NCX-4060 into 50ml of dichloromethane, stir and dissolve completely; mix the above two solutions and add tetrabutyl Ammonium bromide 0.2g, after stirring vigorously for 60min, put it into a separatory funnel, let it stand, and separate the liquid; recover the dichloromethane layer extract, and concentrate the dichloromethane extract to dryness under reduced pressure to recover the unincluded bulk drug; The separated aqueous solution was washed with dichloromethane (20ml×3) and then spray-dried to obtain 180.4g of a loose NCX-4060-sulfobutyl ether-β-cyclodextrin inclusion compound. The inclusion rate was detected by HPLC method to be 86%. NCX-40601.1 g was recovered.
Embodiment 3
[0113] Example 3 Preparation of NCX-4060-sulfobutyl ether-β-cyclodextrin inclusion compound
[0114] Weigh 15g of inclusion material sulfobutyl ether-β-cyclodextrin, add 120ml of distilled water and stir until completely dissolved; add 1.5g of NCX-4060 into 50ml of toluene, stir and dissolve completely; mix the above two solutions and add 0.1g of PEG- 6000, stir vigorously for 60 minutes, transfer to a separatory funnel, stand still, and separate liquids; recover the toluene layer, and concentrate the toluene solution to dryness under reduced pressure to recover unincluded raw materials; the separated aqueous solution is washed with toluene (20ml×3) After spray drying, 15.2 g of loose NCX-4060-sulfobutyl ether-β-cyclodextrin inclusion compound was obtained. The inclusion rate was detected by HPLC method to be 79.2%. 0.21 g of NCX-4060 was recovered.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com