Method for synthesizing sialylated oligosaccharide and analogue thereof
A technology of sialic acid oligosaccharides and analogues, which is applied in the fields of sugar derivatives, organic chemistry, fermentation, etc., can solve the problems of high price and restriction of the synthesis scale of sialic acid oligosaccharides, and achieve the reduction of synthesis costs, good industrial production potential, The effect of the simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1, "One-pot method" synthesis of sialic acid oligosaccharides and their analogs
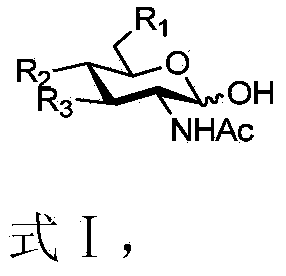
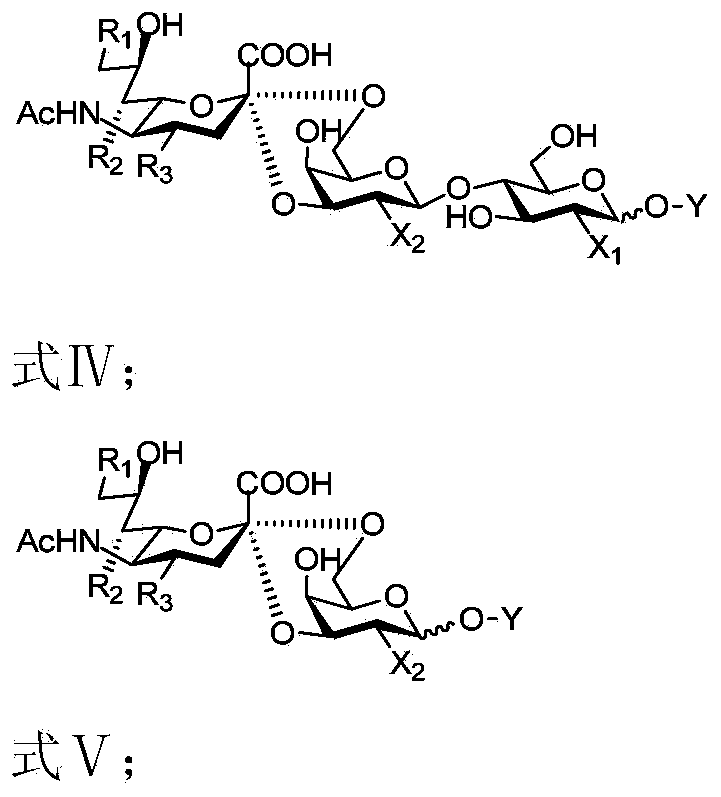
[0060] Using N-acetylneuraminic acid and its analogues (Formula Ⅰ) as starting materials, through the "whole-cell catalysis" and two-step "enzymatic synthesis" methods, without any separation and purification in the middle, the "one-pot method" synthesizes saliva Acid oligosaccharides and their analogs (formula IV or V).
[0061] The specific experimental steps are divided into two steps:
[0062] 1. The donor compound (N-acetylglucosamine, 1 mmol) shown in VI, MgCl 2 (0.025mmol) and pyruvic acid (2mmol) were added to 10ml of engineering bacteria E.coliΔnanTEK / pNA suspension, mixed evenly, reacted in a shaker at 37°C and 200rpm for 16 hours, and centrifuged at 3000g to obtain the supernatant.
[0063] Among them, the bacterial suspension is a solution obtained by suspending the engineering bacteria E.coliΔnanTEK / pNA in 100mM, pH=7.5 Tris HCl buffer, and the OD of E.coliΔnanTEK / pN...
Embodiment 2
[0073] Example 2, "One-pot method" synthesis of sialic acid oligosaccharides and their analogs
[0074] Carry out according to the method for embodiment 1, difference is as follows, all the other are identical:
[0075] Replace α-2,6-sialyltransferase with α-2,3-sialyltransferase (purchased from Tianjin Suntech Pharmaceutical Science and Technology Co., Ltd., Cat.No.:B-03002);
[0076] In step 1), the donor compound, MgCl 2 , Pyruvate and E.coliΔnanTEK / pNA bacterial suspension, the feeding ratio is 0.8mmol:0.02mmol:1.8mmol:10ml;
[0077] In step 2), the feeding ratio of supernatant, receptor compound, cytidine triphosphate, CMP-sialic acid synthase and sialyltransferase is 10ml:0.75mmol:1.45mmol:1.8U:1.8U;
[0078] The compound of formula XVI was obtained in 55% yield.
[0079] The characterization results of the compound shown in product XVI are as follows:
[0080] 1 H NMR (400MHz,D 2 O): δ4.89(1H,d,J2.8Hz),4.35(1H,d,J8.0Hz),4.27(2H,d,J2.4Hz),4.09–3.91(2H,m),3.90–3.72 ...
Embodiment 3
[0082] Example 3, "One-pot method" synthesis of sialic acid oligosaccharides and their analogs
[0083] Carry out according to the method for embodiment 1, difference is as follows, all the other are identical:
[0084] Substituting the acceptor for compound XI as shown,
[0085] In step 1), the donor compound, MgCl 2 , Pyruvate and E.coliΔnanTEK / pNA bacterial suspension, the feeding ratio is 1.2mmol:0.03mmol:2.2mmol:10ml;
[0086] In step 2), the feeding ratio of supernatant, receptor compound, cytidine triphosphate, CMP-sialic acid synthase and sialyltransferase is 10ml:0.85mmol:1.55mmol:2.2U:2.2U.
[0087] The yield of the compound represented by formula XVII was obtained in 52%.
[0088] The characterization results of the compound shown in product XVII are as follows:
[0089] 1 H NMR (400MHz,D 2 O): δ4.63(1H,d,J7.8Hz),4.25(1H,d,J8.0Hz),4.21(2H,d,J2.4Hz),4.01–3.95(2H,m),3.93–3.82 (5H,m),3.76–3.54(9H,m),3.39(1H,t,J8.0Hz),3.29–3.18(2H,m),2.73(1H,dd,J4.5,12.4Hz),2.84 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap