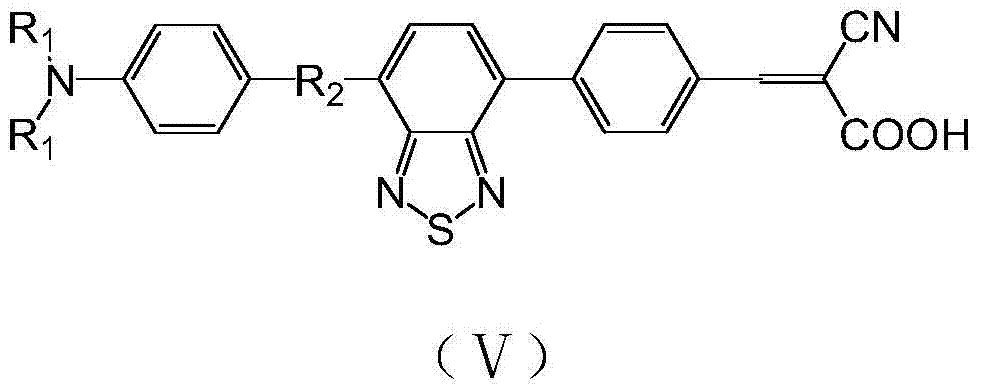
Benzothiadiazole-cyanocinnamic acid receptor-containing organic dye and its use in dye-sensitized solar cell
A technology of cyanophenylacrylic acid and benzothiadiazole, applied in organic dyes, azo dyes, photovoltaic power generation, etc., can solve problems such as narrow spectral response range, achieve easy purification, narrow molecular energy gap, and reduce electronic recombination Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
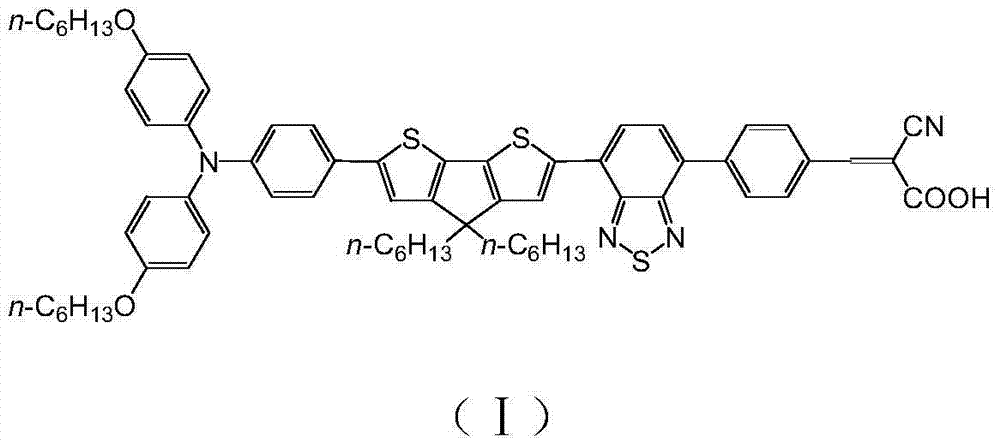
[0018] Example 1: Preparation of organic dye (I) containing benzothiadiazole-cyanophenylacrylic acid acceptor
[0019] The synthetic route is as follows:
[0020]
[0021] Synthetic references for starting material 1 R. Li, J. Liu, N. Cai, M. Zhang, and P. Wang, J. Phys. Chem. B 2010, 114, 4461.
[0022] Synthesis of Intermediate 2:
[0023] Add 6 grams of raw material 1 and 50 ml of tetrahydrofuran into a 100 ml three-necked reaction flask, cool to -78°C under the protection of argon, slowly add 11 ml of n-butyllithium (1.6M) dropwise, and keep the reaction at low temperature after the addition is complete. Hour, add 3.5 grams of trimethyltin chloride at one time, the reaction system is slowly raised to room temperature and stirred for 12 hours, adding 5 milliliters of deionized water to terminate the reaction, the resulting mixed solution is extracted three times with ether, the organic phases are combined, and the The crude product obtained after drying over sodium sul...
Embodiment 2
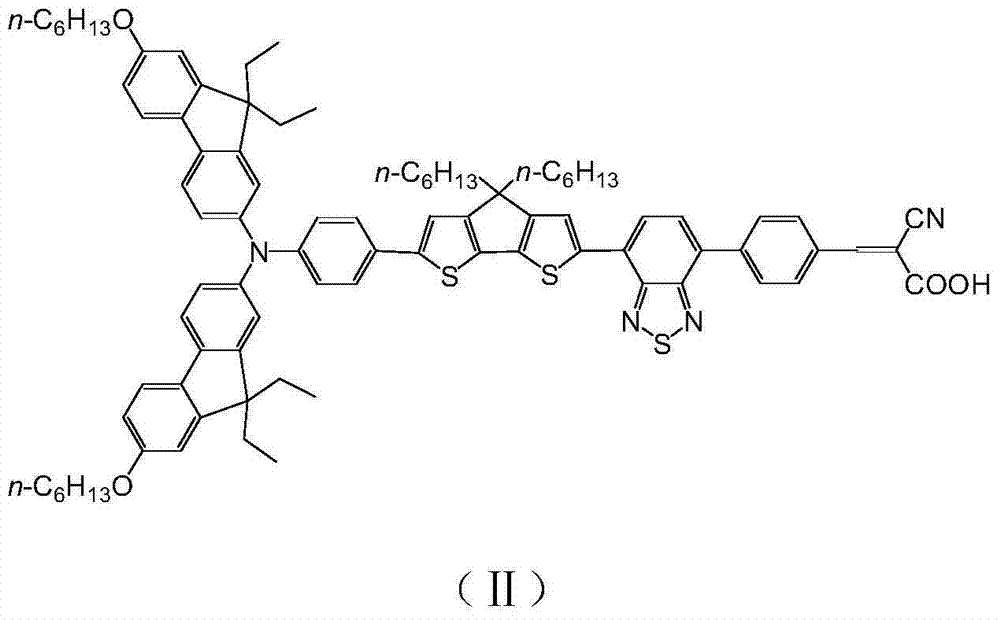
[0045] Example 2: Preparation of organic dye (II) containing benzothiadiazole-cyanophenylacrylic acid acceptor
[0046] The synthetic route is as follows:
[0047]
[0048] References for the synthesis of raw material 4 M. Zhang, Y. Wang, M. Xu, W. Ma, R. Liand P. Wang, Energy Environ. Sci., 2013, 6, 2944.
[0049] Synthesis of Intermediate 5:
[0050] Add 0.24 g of raw material 4 and 4 ml of tetrahydrofuran into a 50 ml three-necked reaction flask, cool to -78°C under the protection of argon, slowly add 0.3 ml of tert-butyllithium (1.3M) dropwise, and keep stirring at low temperature for 3 hours after the addition is completed , then add 70 mg of trimethyl tin chloride, after the dropwise addition, slowly rise to room temperature and stir for 12 hours, then add 20 ml of deionized water to terminate the reaction, and extract the mixed solvent obtained three times with chloroform, combine the organic phases, and use anhydrous Drying over sodium sulfate, the crude product o...
Embodiment 3
[0065] Nano TiO 2 The structured bilayer membrane electrode was soaked in a chloroform solution containing 150 micromoles per liter of dye (Ⅰ) for 12 hours, and then the glass electrode spin-coated with nano-platinum was passed through a 35-micrometer-thick hot-melt ring with TiO2 The structural double-layer film electrode is heated and sealed, and finally the electrolyte is injected into the gap between the two electrodes, which constitutes a dye-sensitized solar cell. The performance structure of the dye-sensitized solar cells obtained by the test is shown in Table 1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap