Preparation and application of hadv chimeric vaccine with influenza virus as carrier
A technology of influenza virus and chimeric vaccine, applied in the direction of virus/bacteriophage, virus, antiviral agent, etc., can solve the problems of not ideal immunization route, low titer of neutralizing antibody, inconvenience, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
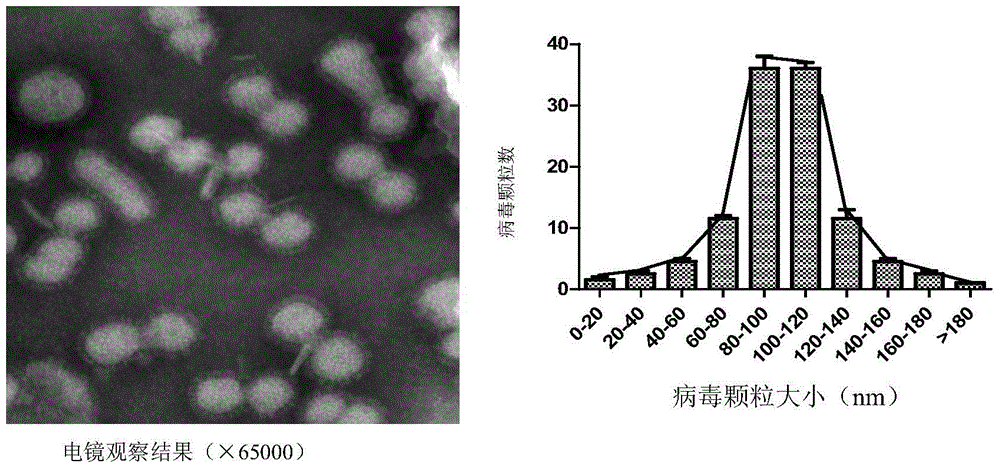
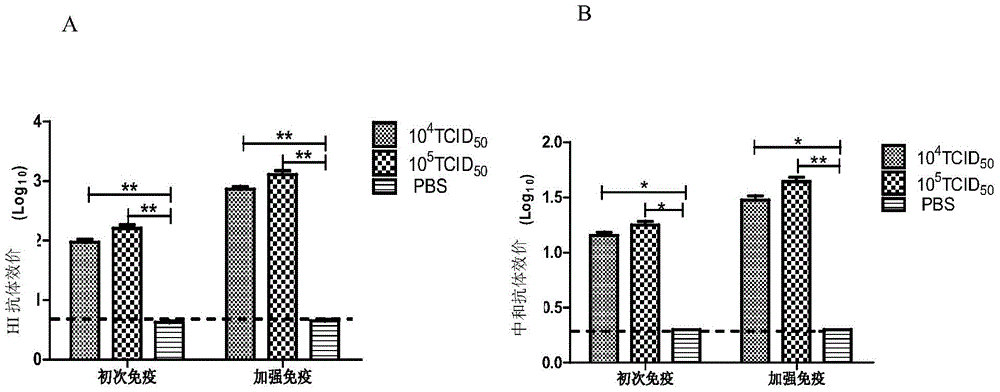
Image
Examples
experiment example 1
[0082] Experimental example 1, the preparation of the HAdV chimeric vaccine rFLU-HAdV / NS1 of the chimeric HAdV-Hexon-L1 / L2 dominant antigen epitope of the influenza virus as the carrier
[0083] 1. The dominant antigenic epitope sequence of Hexon-L1 / L2 (HAdV-Hexon-L1 / L2) in HAdV types 3 and 7 is shown in SEQID No.1, which has been verified by animal experiments and clinical patient serum, and is in line with the next experiment Require.
[0084] 2. Construction of recombinant plasmid pHexon-L1 / L2-NS1
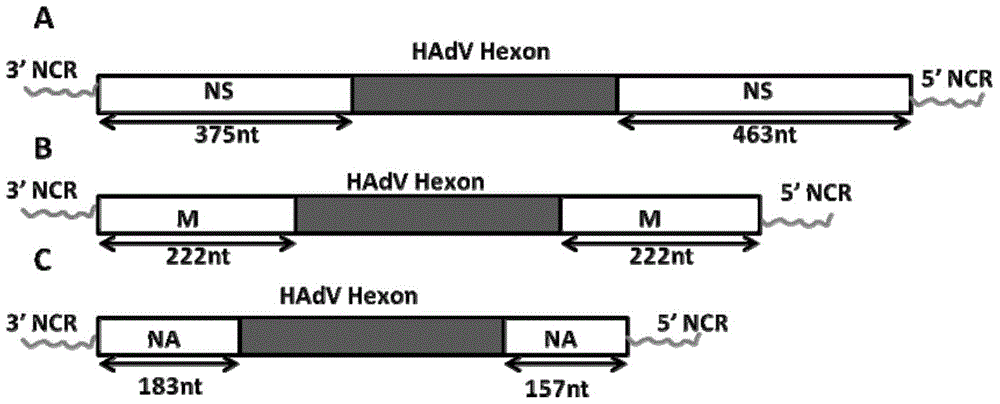
[0085] Using the NS1 gene fragment of the cold-adapted and attenuated influenza virus strain A / AA / 6 / 60 as the target for inserting the dominant epitope gene of HAdV-Hexon-L1 / L2, the recombinant plasmid pHexon-L1 / L2-NS1, the specific policy is as follows figure 1 As shown in A.
[0086] Specifically, the HAdV-Hexon-L1 / L2 dominant antigenic epitope gene is inserted into the coding gene of the first 125 amino acids of the NS1 gene open reading frame (ORF) of the cold-adapted an...
Embodiment 3
[0137] Embodiment 3, the preparation of the HAdV chimeric vaccine rFLU-HAdV / NA of the chimeric HAdV-Hexon-L1 / L2 dominant epitope of the influenza virus as the carrier
[0138] 1. The dominant epitope sequence of HAdV-Hexon-L1 / L2 in HAdV types 3 and 7 is shown in SEQ ID No.1, verified according to the method of step 1 of Example 1, and meets the requirements of the next experiment.
[0139] 2. Construction of recombinant plasmid pHexon-L1 / L2-NA
[0140] Using the NA gene fragment of A / California / 07 / 2009 (H1N1) as the target for inserting the dominant epitope gene of HAdV-Hexon-L1 / L2, the recombinant plasmid pHexon-L1 / L2-NA was constructed by molecular biology methods, specifically Strategies such as figure 1 C shows.
[0141] Specifically, the HAdV-Hexon-L1 / L2 dominant antigen epitope gene was inserted into the first 183 nucleotides and the last nucleotide of the NA gene open reading frame (ORF) of the influenza virus strain H1N1 subtype A / California / 07 / 2009 that year. Betwe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com