PRRSV broad-spectrum neutralizing monoclonal antibody 5d9 and its application
A monoclonal antibody, broad-spectrum technology, applied in the direction of antibodies, applications, antiviral agents, etc., can solve the problems of unreported monoclonal antibody application, lack of neutralizing activity, etc., to prevent virus invasion and protect from infection. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation and identification of monoclonal antibody 5D9
[0026] 1.1 Establishment of hybridoma cell lines
[0027] The PRRSV virus SD16 (provided by the Laboratory of Immunobiology, Northwest Agriculture and Forestry College of Veterinary Medicine) was used as the immunogen, and emulsified with Freund's complete adjuvant (Sigma) 1:1 to immunize 6-week-old female Balb / c mice (provided by Provided by Xi'an Jiaotong University), subcutaneous injection in the abdomen, the dose is (3 × 10^7 PFU / only), booster immunization once every 14 days. Seven days after the third immunization, blood was collected from the tail vein and IFA was used to detect the antibody titer against the immunogen in the serum of the mice. The mice with the best titer were immunized by tail vein injection, and the immunogen was mixed with normal saline 1:1. , the dose is (3×10^7 PFU / only).
[0028] cell fusion
[0029] (1) Aseptically obtain the spleen cells of the immunized mice to ...
Embodiment 2
[0053] Example 2: Determination of neutralizing activity of monoclonal antibody 5D9
[0054] 2.1 Neutralizing activity assay
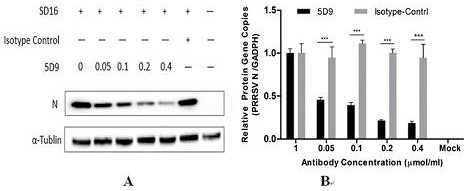
[0055] Virus neutralization experiments were performed using the monoclonal antibody 5D9 to detect its neutralization activity. PAM cells were plated into 24-well plates, and 0.01 MOI of different types of PRRSV SD16 virus were added to each virus. Each virus was added with 100 μg / ml, 200 μg / ml, 300 μg / ml, and 400 μg / ml of antibodies. After incubation at 37°C for 1 h, the cells were replaced. After 36 hours, Western blot and qPCR were performed to confirm that it had neutralizing activity. For specific results, see figure 1 .
[0056] based on figure 1 The results showed that the natural target cells of PRRSV in the host alveolar macrophages (PAMs) were cultured in vitro, and the purified monoclonal antibody 5D9 was used according to the concentration of 0.05, 0.1, 0.2, 0.4 μM / mL (micromoles per milliliter). 0.01M PRRSV-SD16 virus was incubated ...
Embodiment 3
[0062] Example 3: Preparation of monoclonal antibody 5D9 ascites and detection of neutralizing activity
[0063] 3.1 Preparation of ascites
[0064] Mice were sensitized by injection of paraffin oil one week in advance, and the selected hybridoma cells in the logarithmic growth phase were intraperitoneally injected into mice one week later, with 5×10^5 cells per mouse. The ascites was collected 7 days after the injection of hybridoma cells. The removed ascites was centrifuged at 5000 g × 10 min at 4°C, and the supernatant ascites was aspirated and collected in an EP tube, and stored at -20°C.
[0065] Detection of Neutralizing Activity of Monoclonal Antibody 5D9 in Ascites
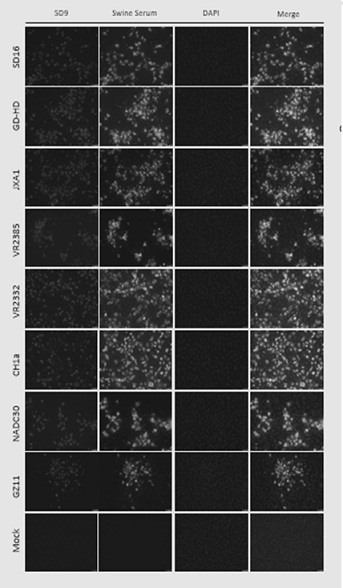
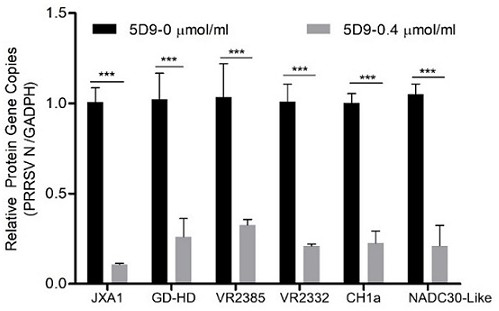
[0066] Using the ascites fluid containing the monoclonal antibody, a virus neutralization experiment was performed by a 4-fold dilution method to detect its neutralization activity. PAM cells were plated to a 24-well plate, and 0.01MOI PRRSV-SD16 virus (PRRSV-II type) or PRRSV-GZ11 (PRRSV-I type virus) w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com