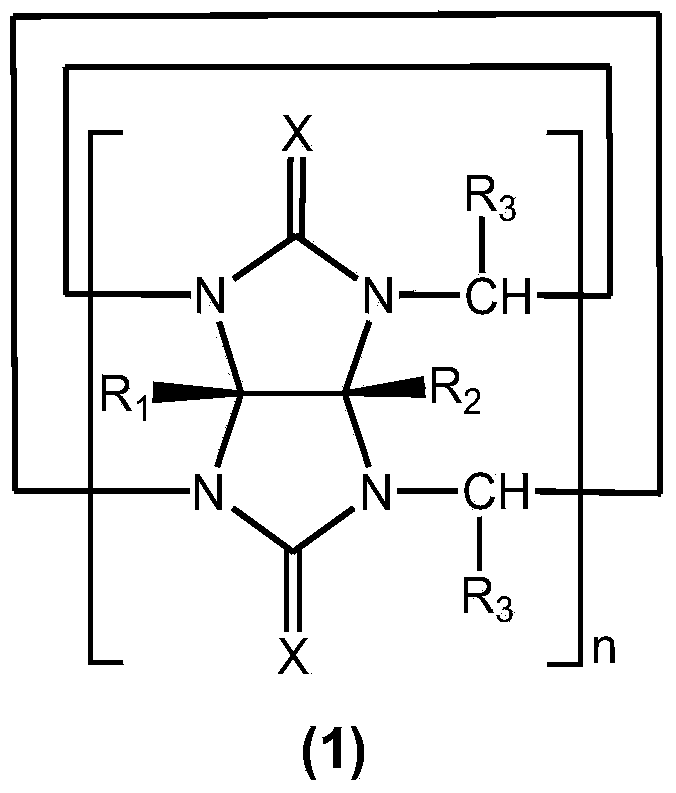
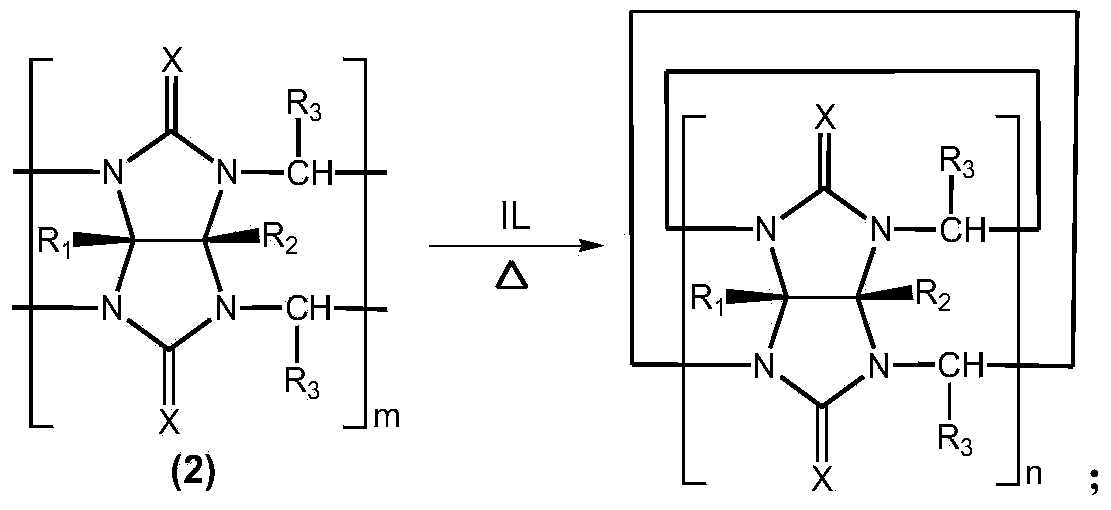
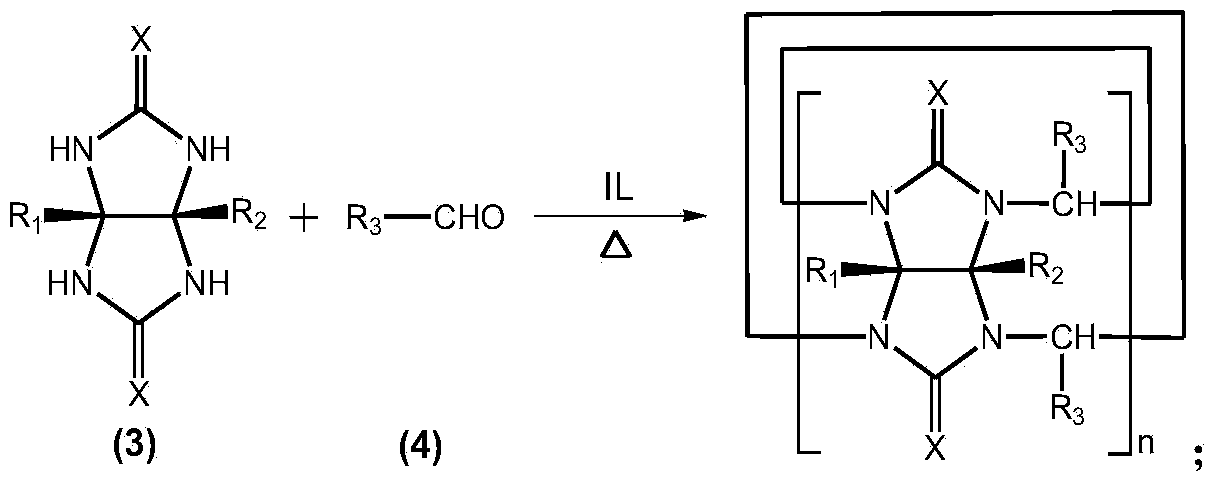
Method for preparing cucurbituril and cucurbituril derivative
A technology of cucurbituril and derivatives, which is applied in the field of preparation of cucurbituril and cucurbituril derivatives, can solve the problems of corrosiveness, high equipment requirements, environmental pollution, etc., and achieve strong corrosiveness, serious acid pollution, and low yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Glycoluril-formaldehyde polymer (0.250g), 1-methyl-3-(3-sulfonic acid) propylimidazolium bisulfate ([C 3 SO 3 Hmim] HSO 4 ) ionic liquid (1.000g) and water (2.000g) were mixed, heated to 160° C. for 30 minutes by microwave (800W). After the reaction solution was left to stand for 1-2 days, the cucurbit [6] urea product crystallized (0.070 g), and the ionic liquid could be recycled.
Embodiment 2
[0031] Glycoluril-formaldehyde polymer (0.250g), 1-methyl-3-(3-sulfonic acid) propylimidazole tetrafluoroborate ([C 3 SO 3 Hmim] BF 4 ) ionic liquid (1.000g) and water (2.000g) were mixed, heated to 160° C. for 30 minutes by microwave (800W). After the reaction solution was cooled, a large amount of cucurbit [6] urea product crystallized (0.141 g), and the ionic liquid could be recycled.
Embodiment 3
[0033] Glycoluril-formaldehyde polymer (0.250g), 1-methyl-3-(3-sulfonic acid) propylimidazolium bisulfate ([C 3 SO 3 Hmim] HSO 4 ) ionic liquid (1.000g) and water (2.000g) were mixed, heated to 160° C. for 30 minutes by microwave (800W). The reaction solution was poured into methanol to form a white precipitate, which was filtered and vacuum-dried to obtain 0.238 g of cucurbituril product, with a yield of 95.2%, in which cucurbit[5]uril, cucurbit[6]uril and cucurbit[7]uril each accounted for 12% , 57% and 31%. After the filtrate is dried, the ionic liquid is recycled.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com