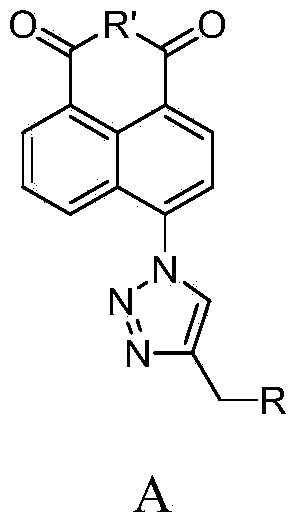
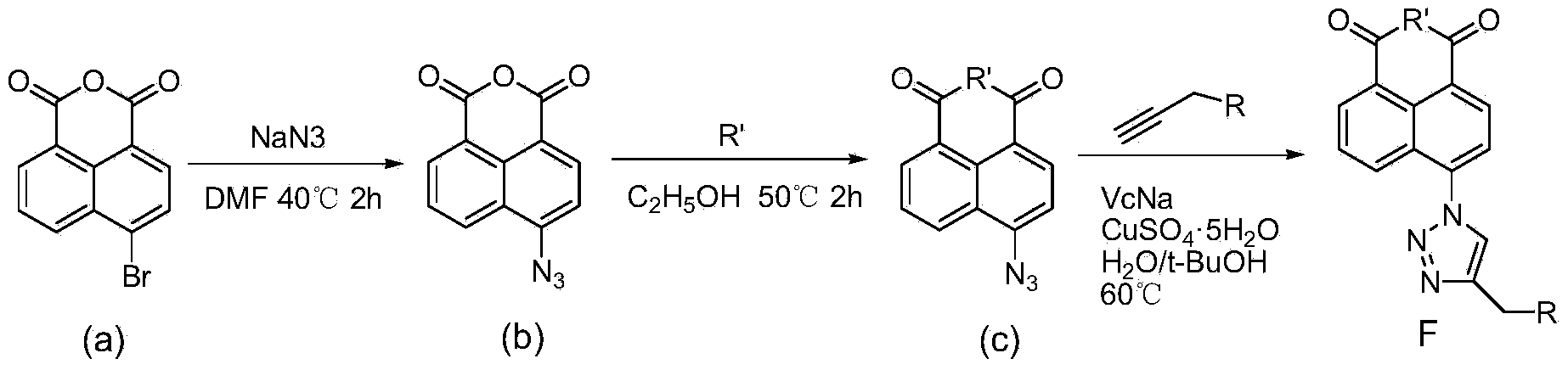
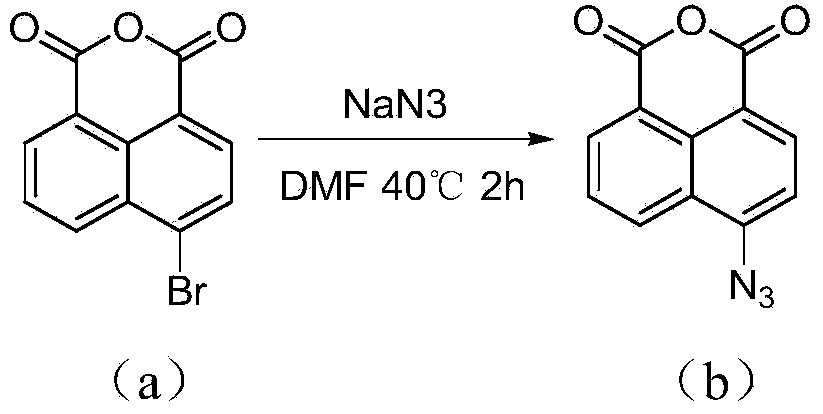
Synthesis of naphthalene nucleus 4-position 1,2,3-triazole containing naphthalimide derivative and application thereof
A technology of naphthalimide and derivatives, applied in the field of synthesis of naphthalimide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] N-(N',N'-Dimethylaminoethyl)-4-(4-morpholinomethyl-[1,2,3]-triazole)-1,8-naphthalimide (F1 )Synthesis
[0027] ①In a 50mL two-necked bottle, add 2.50g of 4-bromo-1,8-naphthalene anhydride and 35mL of DMF, stir well at room temperature, and dissolve 0.88g of NaN 3 Dissolve in 2 mL of water, drop into the reaction system, heat to 40°C for 2 hours, pour into cold water after standing, filter with suction, wash with water, and dry to obtain 2.01 g of compound (b) as a yellow-green solid with a yield of 93%.
[0028]
[0029] ②In a 50mL two-necked bottle, add 1g of compound (b) and 30mL of absolute ethanol, stir well at room temperature, add 0.6mL of N,N-dimethylethylenediamine into the reaction system, heat to 50°C for 2h, and statically Pour it into cold water, filter with suction, wash with water, and dry to obtain 1.17 g of compound (c) as a light yellow solid with a yield of 96%.
[0030]
[0031] ③In a 25mL two-necked bottle, add 0.62g compound (c), 188μL 3-mor...
Embodiment 2
[0036] N-(N',N'-dimethylaminoethyl)-4-(4-thiomorpholinomethyl-[1,2,3]-triazole)-1,8-naphthalimide ( Synthesis of F2)
[0037] Except that 3-thiomorpholinopropyne was used instead of 3-morpholinopropyne, other synthesis and purification methods were the same as in Example 1 to obtain the target product F2 as a light yellow solid with a yield of 70%. m.p.: 143.4-144.0°C.
[0038]
[0039] 1 H NMR (400MHz, CDCl 3 )δ8.72(d, J=7.2Hz, 2H), 8.27(d, J=8.8Hz, 1H), 7.97(s, 1H), 7.93–7.77(m, 2H), 4.39(t, J=6.3 Hz,2H),3.89(s,2H),2.90(t,4H),2.75(t,2H),2.74(t,4H),2.42(s,6H).
[0040] TOF MS (m / z): C 23 h 27 N 6 o 2 S+, Calculated: 451.1916, Found: 451.1918.
Embodiment 3
[0042] N-(N',N'-dimethylaminoethyl)-4-(4-piperidinylmethyl-[1,2,3]-triazole)-1,8-naphthalimide (F3 )Synthesis
[0043] Except that 3-morpholinopropyne was replaced by 3-piperidylpropyne, other synthesis and purification methods were the same as in Example 1, and the target product F3 was obtained as a yellow solid with a yield of 76%. m.p.: 142.5-144.2°C.
[0044]
[0045] 1 H NMR (400MHz, CDCl 3)δ8.71(d, J=7.7Hz, 2H), 8.30(d, J=8.5Hz, 1H), 8.10(s, 1H), 7.89–7.81(m, 2H), 4.37(t, J=6.9 Hz, 2H), 3.89(s, 2H), 2.70(t, J=6.9Hz, 2H), 2.64(t, 4H), 2.37(s, 6H), 1.74–1.63(m, 4H), 1.57–1.44 (m,2H).
[0046] 13 C NMR (101MHz, CDCl 3 )δ163.65,163.13,145.18,138.18,132.26,130.70,129.51,129.09,128.59,126.36,125.04,123.80,123.44,122.92,56.90,54.87,54.05,45.938.75,28
[0047] TOF MS (m / z): C 24 h 29 N 6 o 2 +, calculated value: 433.2352, measured value: 433.2334.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com