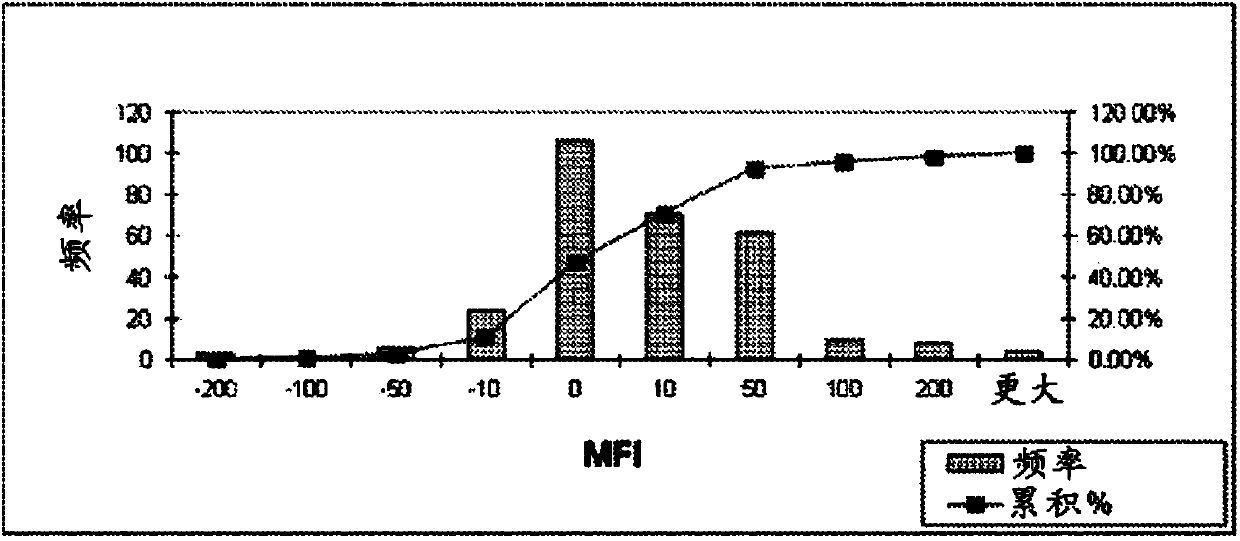
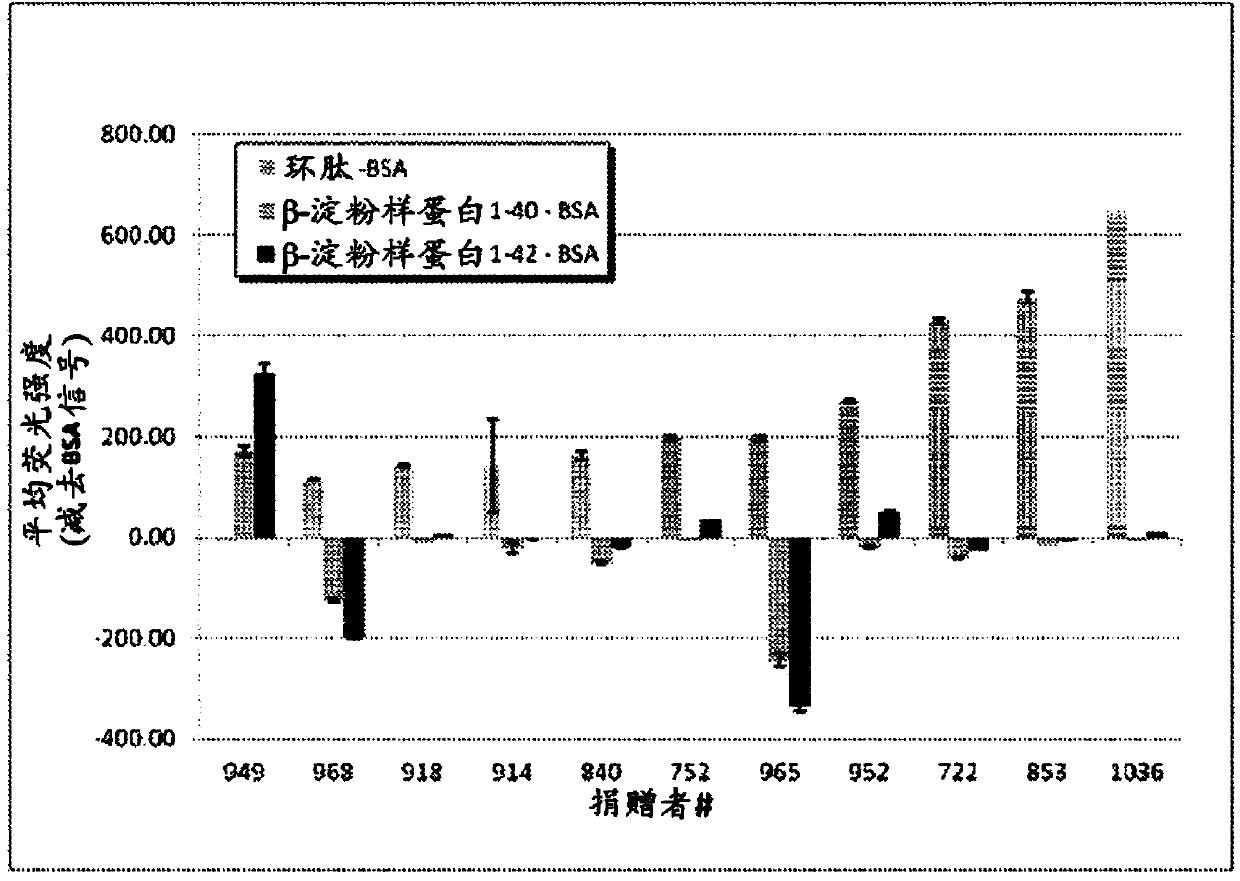
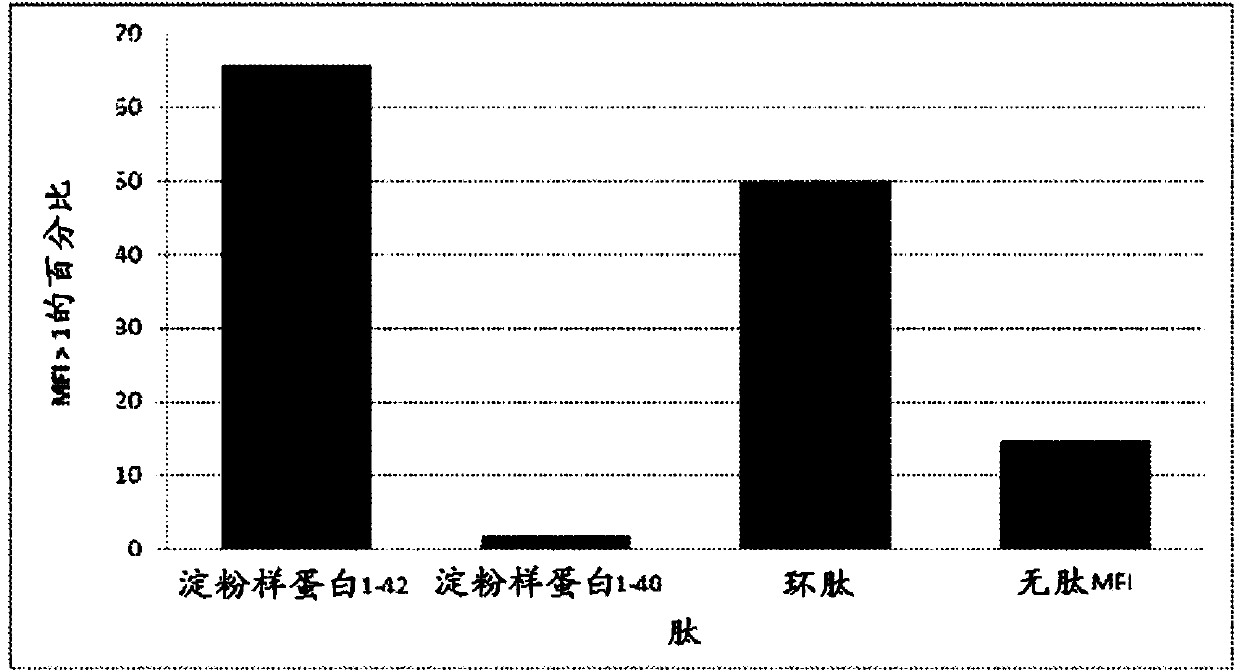
Compositions and methods for treating alzheimer's disease
A technology for Alzheimer's disease and biological samples, applied in chemical instruments and methods, drug combinations, nervous system diseases, etc., can solve problems such as poor transformation of mice
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0137] Embodiment 1: cyclic peptide and Coupling of Microspheres
[0138] Various peptides derived from Αβ and microsphere coupling, Superparamagnetic carboxylated Microspheres. Αβ(1-42) and Microsphere 26 area coupling, Αβ(1-40) is coupled to microsphere 28 area, cyclic peptide cSNK (sequence: CGSNKGC) coupled with BSA is coupled to microsphere 18 area, and BSA is coupled to microsphere 30 area couplet. Αβ(1-42) and Αβ(1-40) were purchased from Bachem (Torrance, CA). Cyclic peptides were provided by the laboratory of Dr. Neil Cashman, Brain Research Center (Vancouver, BC).
[0139] The cyclic peptide comprises an amino acid sequence with at least SNK, a conformational epitope corresponding to the solvent-exposed, antibody-accessible knuckle region of oligomeric Aβ described in PCT application PCT / CA2011 / 000238.
[0140] Microspheres (regions 018, 026, 028 and 030) were obtained from Luminex Corporation (Austin, TX). 1.25×10 as required 6 The 1× scale coupling...
Embodiment 2
[0141] Example 2: Collection of plasma samples
[0142] 295 plasma samples from 295 donors were tested in the study. All donors were adults residing in Canada ranging in age from 18 to 75 years. Donors consisted of 159 men and 136 women. Plasma samples were collected between April 15, 2010, and February 15, 2011.
Embodiment 3
[0145] Example 3: multiplex immunoassay
[0146] The conjugated microspheres described in Example 1 were immunoassayed on collected plasma samples.
[0147] Each assay was performed by diluting the peptide-coated microspheres in PBS-T so that when 100 [mu]L was added to each well of a 96-well microplate, 3000 microspheres of each microsphere type were present per well. After adding the microspheres to the microplate, 50 μL each of the diluted plasma samples were added to the microspheres. The IVIG product was used as a control for each assay and 50 μL of the IVIG product (1 in 100 dilution in PBS-T) was diluted and added to the remaining wells. The mixture of plasma samples and peptide-coated microspheres was incubated at room temperature on a plate shaker in the dark for 60 minutes. Remove unbound antibody and remaining matrix by placing the microplate on a magnetic separator plate for 1 min, then pouring the supernatant into a sink by inverting the microplate attached to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com