Clonal strains of attenuated vaccinia viruses and methods of use thereof
A virus strain, virus strain technology, applied in the direction of viruses, antiviral agents, viruses/phages, etc., can solve problems such as reducing and weakening the oncolytic properties of viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0071] 4. Preparation of the virus
[0072] F. Pharmaceutical compositions, combinations and kits
[0073] 1. Pharmaceutical composition
[0074] 2. Host cell
[0075] 3. Combination
[0076] 4. Kit
[0077] G. Methods of treatment, diagnosis and monitoring
[0078] 1. Treatment
[0079] 2. Diagnosis and monitoring methods
[0080] 3. Application
[0081] a. Steps before applying the virus
[0082] b. Application mode
[0083] c. Dosage and dosing schedule
[0084] d. Co-administration
[0085] i. Application of multiple viruses
[0086] ii. Therapeutic compounds
[0087] iii. Immunotherapy and biological therapy
[0088] e. Object status
[0089] 4. Monitoring
[0090] a. Monitoring viral gene expression
[0091] b. Monitor tumor size
[0092] c. Monitor antibody titer
[0093] d. Monitoring general health diagnosis
[0094] e. Monitoring co-treatment
[0095] H. Examples
[0096] A. Definition
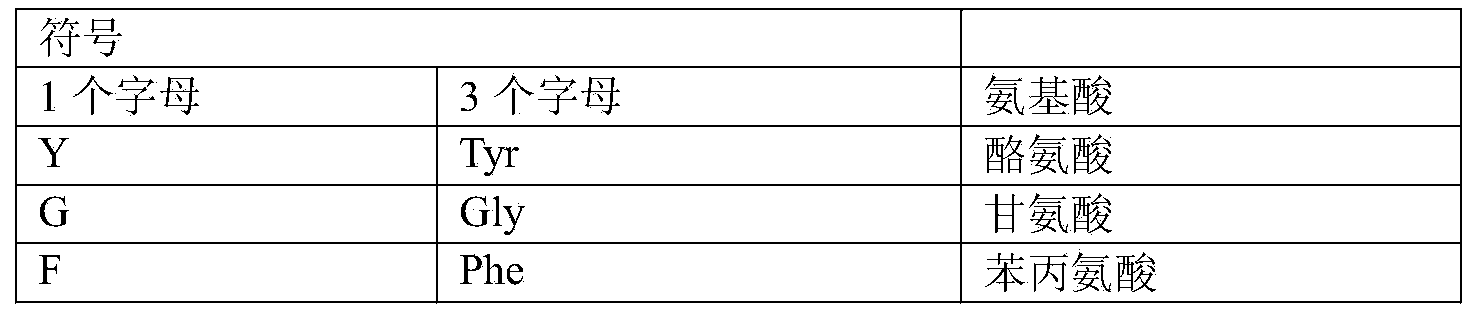
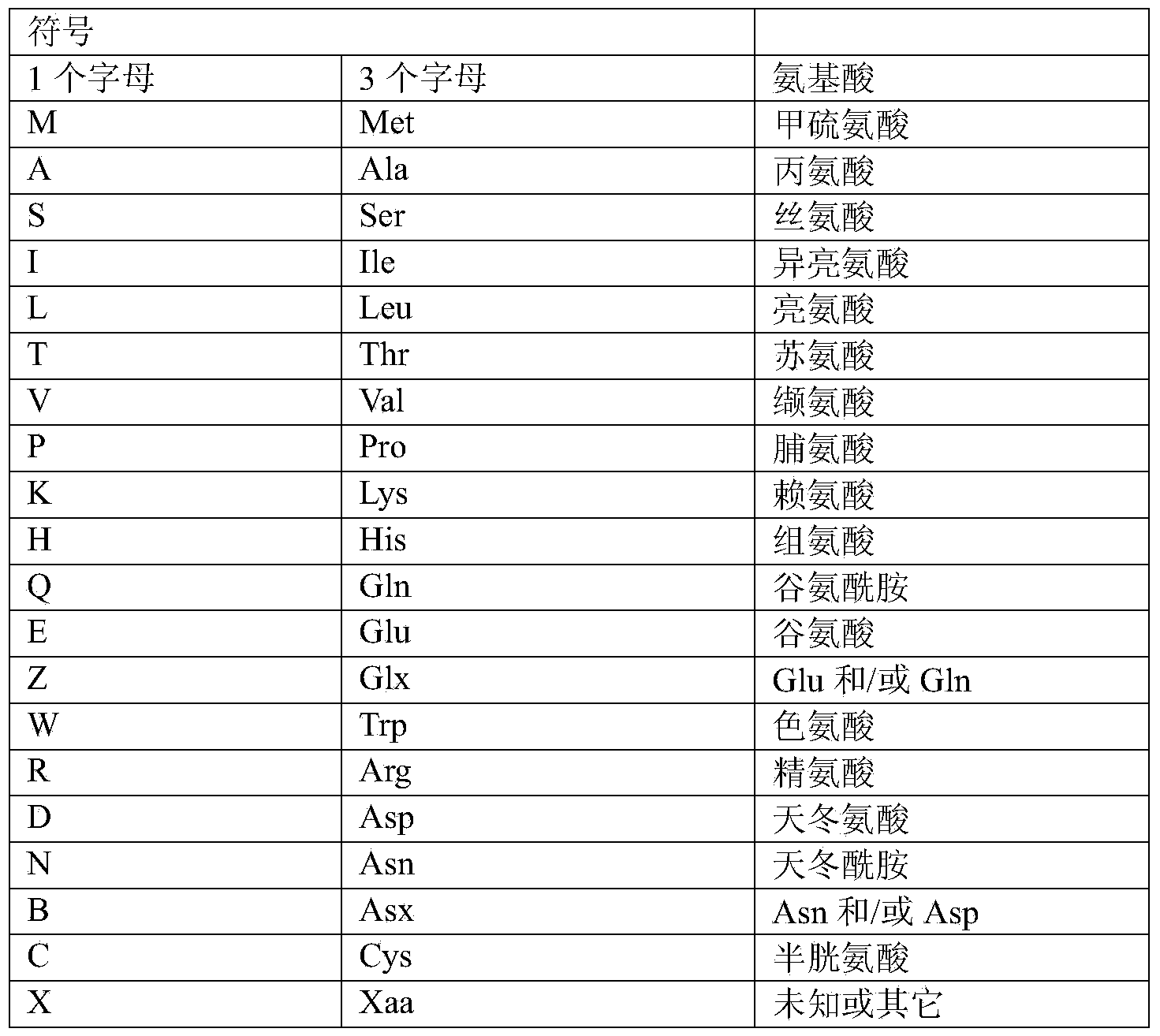
[0097] Unless otherwise specified, all technical and academic terms used in the present invention have the same...
Embodiment 1
[0564] Example 1: Isolation of LIVP clone isolate
[0565] Plate African green monkey kidney fibroblasts CV-1 cells (ATCC No. CCL-70; American Type Culture Collection (Manassas, VA)) on a 6-well plate with a density of 5×10 5 Cells / well, in Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% antibiotic-antifungal solution (Mediatech, Inc., Herndon, VA) and 10% fetal bovine serum (FBS, Mediatech, Inc., Herndon, VA) , Mediatech, Inc., Herndon, VA) 5% CO at 37℃ 2 Grow overnight in a humidified incubator. When the cells reach 90% confluence, CV-1 cells are diluted 10-fold with vaccinia virus LIVP (vaccinia virus strain, originally produced by adapting Lister strain (ATCC Catalog No.VR-1549) on calf skin) (Moscow Institute of Virus Preparation, Russia, Al'tshtein et al., (1983) Dokl. Akad. Nauk USSR285: 696-699)) infection. Use serial dilutions to infect to ensure the separation of fully separated plaques. Two days after infection, 8 well-separated plaques that showed a l...
Embodiment 2
[0566] Example 2: In vitro infection of various normal and cancer cell lines
[0567] Analyze the growth of LIVP clone isolates in various normal and cancer cell types, and compare them with the parent LIVP strain, two LIVP-derived strains GLV-1h68 (see U.S. Patent No. 7,588,767) and GLV-1h74 (see U.S. Patent Application Publication No. 2009-0098529 and 2009-0053244) and the growth of vaccinia virus WR (ATCC Catalog No. VR-1354) were compared. GLV-1h68 contains a DNA insertion in the locus of the LIVP strain (SEQ ID NO: 9). GLV-1h68 contains expression cassettes encoding detectable marker proteins at F14.5L (also called F3 in LIVP), thymidine kinase (rK) and hemagglutinin (HA) loci. Contains early / late promoter P which is synthesized by vaccinia virus SEL ((P SEL ) Ruc-GFP)-controlled Ruc-GFP cDNA molecule (a fusion of DNA encoding Renilla luciferase and DNA encoding GFP) is inserted into the F14.5L locus; contains the early / late start of vaccinia virus Child P 7.5k ((P 7.5k ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com