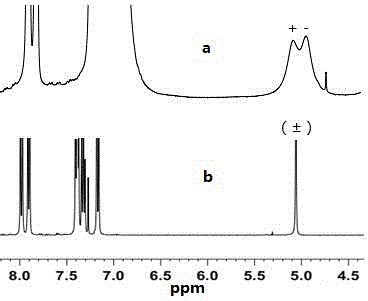
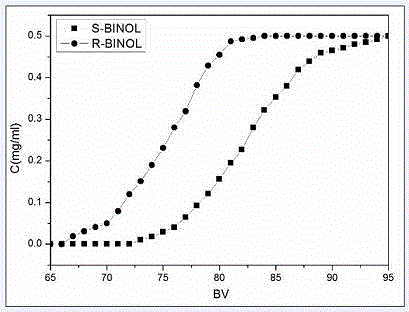
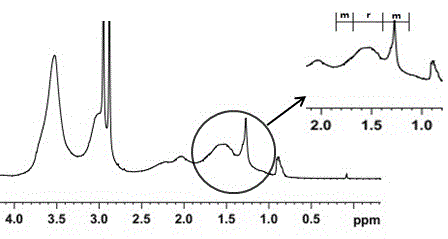
Method for environmentally preparing novel material capable of splitting chiral binaphthol from L-phenylalanine
A technology of phenylalanine methyl ester and polymethacryloyl phenylalanine methyl ester, which is applied in the field of material preparation, can solve problems such as poor environmental compatibility, high raw material cost, and poor operability, and achieve easy operation, Effects of increased yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Preparation of N-methacryloyl-L-phenylalanine methyl ester (A-L-M) under ionic liquid
[0049] Slowly add 2.5g of thionyl chloride into the mixed solution of 15g of methanol and 1.3g of L-phenylalanine in an ice bath at 0°C. After stirring for 2 hours, raise the temperature to 65°C and stir for 2 hours to obtain a mixed solution. Afterwards, the obtained 1.5 g of L-phenylalanine methyl ester hydrochloride was added to 38 g of ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate containing 6 g of triethylamine. Slowly add 3 g of methacryloyl chloride dropwise in an ice bath at 0°C, stir for 1 h, then move to room temperature and stir for 2 h to obtain a mixed solution, which is extracted, dried, and rotary evaporated to obtain the chiral monomer A-L-M;
[0050] (2) Preparation of the material poly(A-L-M) which can detach chiral binaphthol under ionic liquid
[0051] Add 0.3 g of A-L-M and 0.03 g of yttrium trifluoromethanesulfonate prepared in step (1) to 2 g of i...
Embodiment 2
[0053] (1) Preparation of N-methacryloyl-L-phenylalanine methyl ester (A-L-M) under ionic liquid
[0054] Slowly add 6g of thionyl chloride into the mixed solution of 60g of methanol and 2g of L-phenylalanine in an ice bath at 0°C. After stirring for 2.5h, raise the temperature to 65°C and stir for 2.5h to obtain a mixed solution, which is then rotary evaporated Afterwards, the obtained 2 g of L-phenylalanine methyl ester hydrochloride was added to 53 g of ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate containing 12 g of triethylamine. Slowly add 4.5g of methacryloyl chloride dropwise in an ice bath at 0°C, stir for 2 hours, then move to room temperature and stir for 3 hours to the mixed solution, extract, dry, and rotary evaporate to obtain the chiral monomer A-L-M;
[0055] (2) Preparation of the material poly(A-L-M) which can detach chiral binaphthol under ionic liquid
[0056] Add 0.5 g of A-L-M and 0.075 g of yttrium trifluoromethanesulfonate prepared in step (1)...
Embodiment 3
[0058] (1) Preparation of N-methacryloyl-L-phenylalanine methyl ester (A-L-M) under ionic liquid
[0059] Under an ice bath at 0°C, 24g of thionyl chloride was slowly added to a mixed solution of 80g of methanol and 4g of L-phenylalanine, stirred for 3 hours, raised to 65°C and stirred for 5 hours to obtain a mixed solution, which was obtained after rotary evaporation 5g of L-phenylalanine methyl ester hydrochloride was added in 90g of ionic liquid 1-ethyl-3-methylimidazole ethyl sulfate containing 40g of triethylamine. Slowly add 15g of methacryloyl chloride dropwise in an ice bath at 0°C, stir for 3 hours, then move to room temperature and stir for 5 hours to obtain a mixed solution, which is extracted, dried, and rotary evaporated to obtain the chiral monomer A-L-M;
[0060] (2) Preparation of the material poly(A-L-M) which can detach chiral binaphthol under ionic liquid
[0061] Add 0.8 g of A-L-M and 0.16 g of yttrium trifluoromethanesulfonate prepared in step (1) to 6 g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com