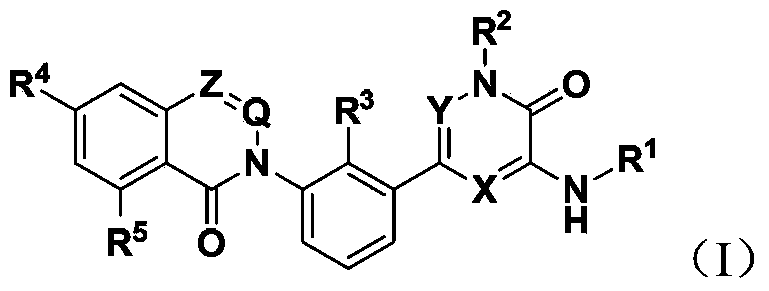
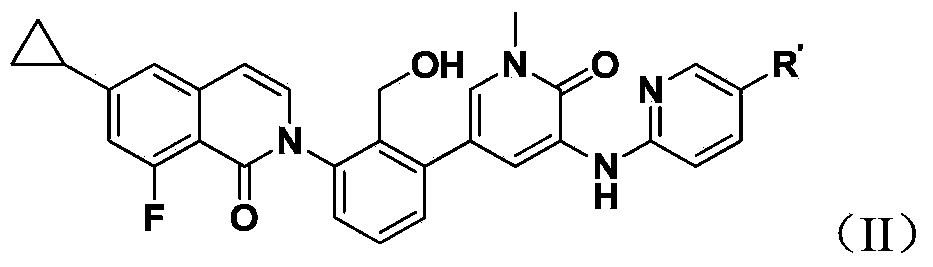
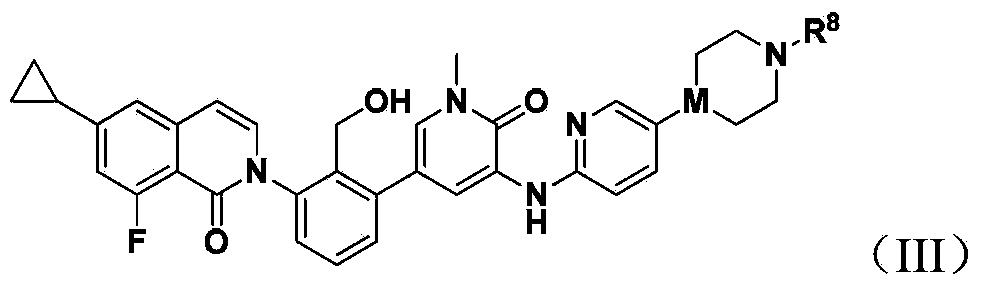
Bruton's kinase inhibitor
A C1-C6, selected technology, applied in the field of new derivatives, can solve the problems of easy metabolism and low exposure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Embodiment 1: the preparation of compound 1
[0110]
[0111] Preparation of tert-butyl 4-(6-nitro-3-pyridyl)-1-piperazine-1-carboxylate
[0112] Weigh 5-bromo-2-nitropyridine (17.1g, 84.7mmol) and dimethyl sulfoxide (DMSO) (550mL) into a 1L three-necked flask, add N-tert-butoxycarbonylpiperazine (15.8 g, 84.7mmol) and potassium carbonate (K 2 CO 3 ) (35.4g, 254.1mmol), after the addition was completed, the temperature was raised to 65°C to react overnight, cooled to room temperature, and the reaction solution was slowly poured into 2L of ice-water mixture and stirred for 30 minutes. A large amount of solids were precipitated, filtered and dried in vacuo to obtain Yellow product (14g, 53.8%). MS(ESI)m / z:[M+H] + =309.2. 1 H-NMR (CDCl 3 ,400MHz): δ8.18(d,1H),8.13(s,1H),7.16(d,1H),3.64(t,4H),3.45(t,4H),1.49(s,9H)ppm.
[0113] Preparation of tert-butyl 4-(6-amino-3-pyridyl)-1-piperazine-1-carboxylate
[0114]Weigh tert-butyl 4-(6-nitro-3-pyridyl)-1-piperazine-1-c...
Embodiment 2
[0125] Embodiment 2: the preparation of compound 2
[0126]
[0127] Preparation of tert-butyl-6-nitro-5',6'-dihydro-[3,4'-bipyridine]-1'(2'H)-carboxylate
[0128] Weigh 5-bromo-2-nitropyridine (250mg, 1.23mmol), N-tert-butoxycarbonyl-3,6-dihydro-2H-pyridine-4-boronic acid pinacol ester (456mg, 1.47mmol), Pd (dppf) 2 Cl 2 ·CH 2 Cl 2 (30mg, 0.37mmol) was placed in a 50mL three-necked flask, replaced with argon three times, added 1,4-dioxane (15mL) and stirred for 5 minutes, then added 2M NaHCO 3 (1.85mL, 3.7mmol) was replaced with argon three times, then heated to 100°C for 2 hours, stopped the reaction and cooled to room temperature, filtered with diatomaceous earth, added water and ethyl acetate (20mL*3) to the filtrate, and then Wash once with saturated NaCl (10mL), and finally wash the ester layer with anhydrous NaCl 2 After drying with SO4, the product was separated by column chromatography (270 mg, 71%), MS (ESI) m / z: [M+H] + =306.2. 1 H-NMR (CDCl 3 , 400MHz):...
Embodiment 3
[0141] Example 3: In vitro biochemical level inhibition of protein kinase (PK) activity experiment
[0142] Materials and methods: BTK kinase, from Invitrogen; HTRF KinEASE; TK kit (Cisbio); 384-well plate (Greiner); ATP (sigma), MgCl 2 (sigma) company; PHERAstar FS multifunctional microplate reader (BMG company); low-speed centrifuge (StaiteXiangyi company); incubator (Binder company). The selected positive drug is RN486, which can be prepared by referring to existing literature reports in this field (WO2010100070), and the structure is as follows:
[0143]
[0144] Compound dissolution and storage: Depending on the solubility, the test compound is prepared into a 0.5-10mmol / L mother solution with DMSO, and stored at -20°C after aliquoting;
[0145] Preparation of compound working solution: Before testing, take out the aliquoted compound from the refrigerator, dilute it to 50× required concentration with pure DMSO; then dilute the compound to 4× required concentration wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com