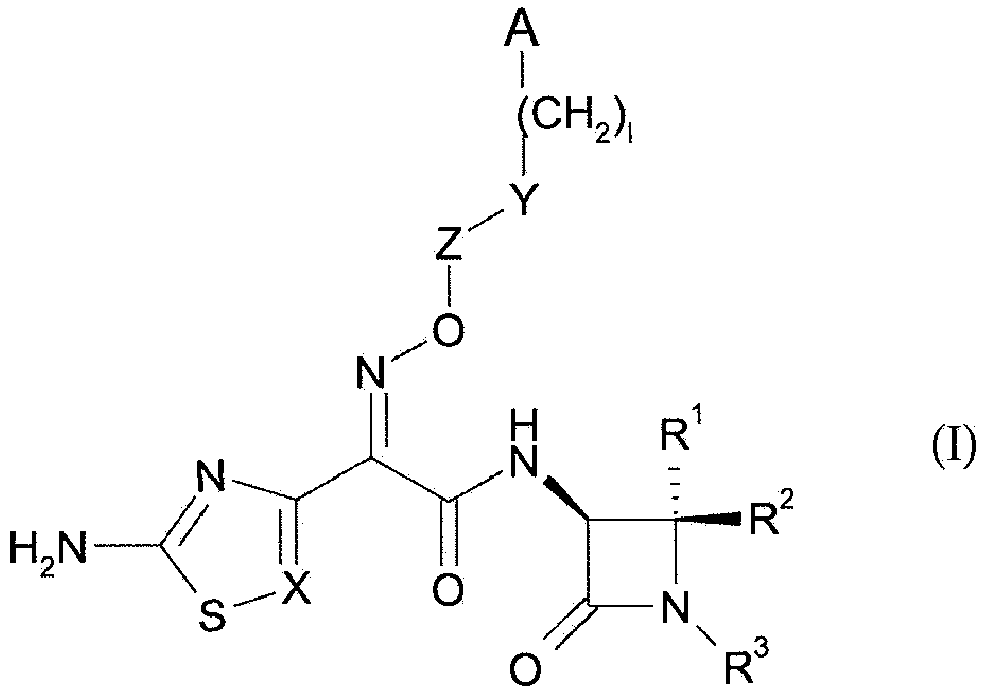
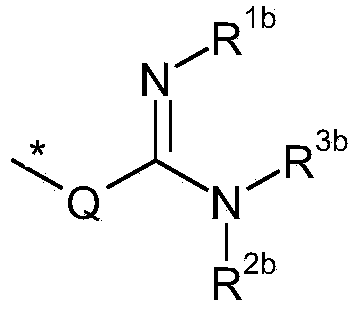
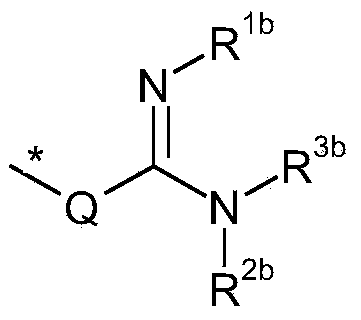
Amidine substituted beta - lactam compounds, their preparation and use as antibacterial agents
A compound and substituent technology, applied in the field of new β-lactam compounds, can solve problems that endanger the mid-term availability of existing compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1103] (2Z)-2-(2-Amino-1,3-thiazol-4-yl)-2-{[2-(4-carbamimidino-3-hydroxyphenoxy)ethoxy]imino}- N-[(3S)-2,2-Dimethyl-4-oxo-1-(sulfoxy)azetidin-3-yl]acetamide
[1104] 1 H NMR (400MHz, DMSO-d 6 ):δ=1.23(s,3H),1.40(s,3H),4.19(m,2H),4.38(m,2H),4.59(d,J=8.0Hz,1H),6.49(s,1H) ,6.57(d,J=8.8Hz,1H,),6.76(s,1H),7.21(br s,2H),7.57(d,J=8.8Hz,1H),9.49(d,J=8.0Hz, 1H).
[1105] MS (ES + ) m / z: C 19 h 24 N 7 o 9 S 2 [M+H] of + Calculated value: 558.11. Experimental value: 558.14.
[1106] HPLC: 98.63%
Embodiment 2
[1108] (2S,3S)-3-{[(2Z)-2-(2-Amino-1,3-thiazol-4-yl)-2-{[2-(4-formamido-3-hydroxyphenoxy base)ethoxy]imino}acetyl]amino}-2-methyl-4-oxazetidine-1-sulfonic acid
[1109] 1 H NMR (400MHz, DMSO-d 6 ):δ=1.33(d,J=6.2Hz,3H),3.67(m.,1H),4.22(m,2H),4.35(m,2H),4.41(m,1H),6.45(br.s , 1H,), 6.52 (m, 1H), 6.79 (s, 1H), 7.22 (m, 2H), 7.56 (d, J=8.8Hz, 1H), 9.32 (m, 1H).
[1110] MS (ES - ) m / z: C 18 h 20 N 7 o 8 S 2 [M-H] - Calculated value: 526.08. Experimental value: 526.13.
[1111] HPLC: 91.43%
Embodiment 3
[1113] (2S,3S)-3-{[(2Z)-2-(2-Amino-1,3-thiazol-4-yl)-2-({2-[(6-carbamidinopyridin-3-yl )oxy]ethoxy}imino)acetyl]amino}-2-methyl-4-oxazetidine-1-sulfonic acid
[1114] 1 H NMR (400MHz, DMSO-d 6 ):δ=1.35(d,J=6.2Hz,3H),3.66(dd,J=6.0 and 2.5Hz,1H),4.26-4.57(m,5H),6.78(s,1H),7.76(dd, J=8.9 and 2.7Hz, 1H), 8.26(d, J=8.9Hz, 1H), 8.52(d, J=2.3Hz, 1H), 8.95(s, 2H), 9.21-9.49(m, 3H).
[1115] MS (ES - ) m / z: C 17 h 20 N 8 o 7 S 2 [M-H] - Calculated value: 512.09. Experimental value: 512.53.
[1116] HPLC: 96.60%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com