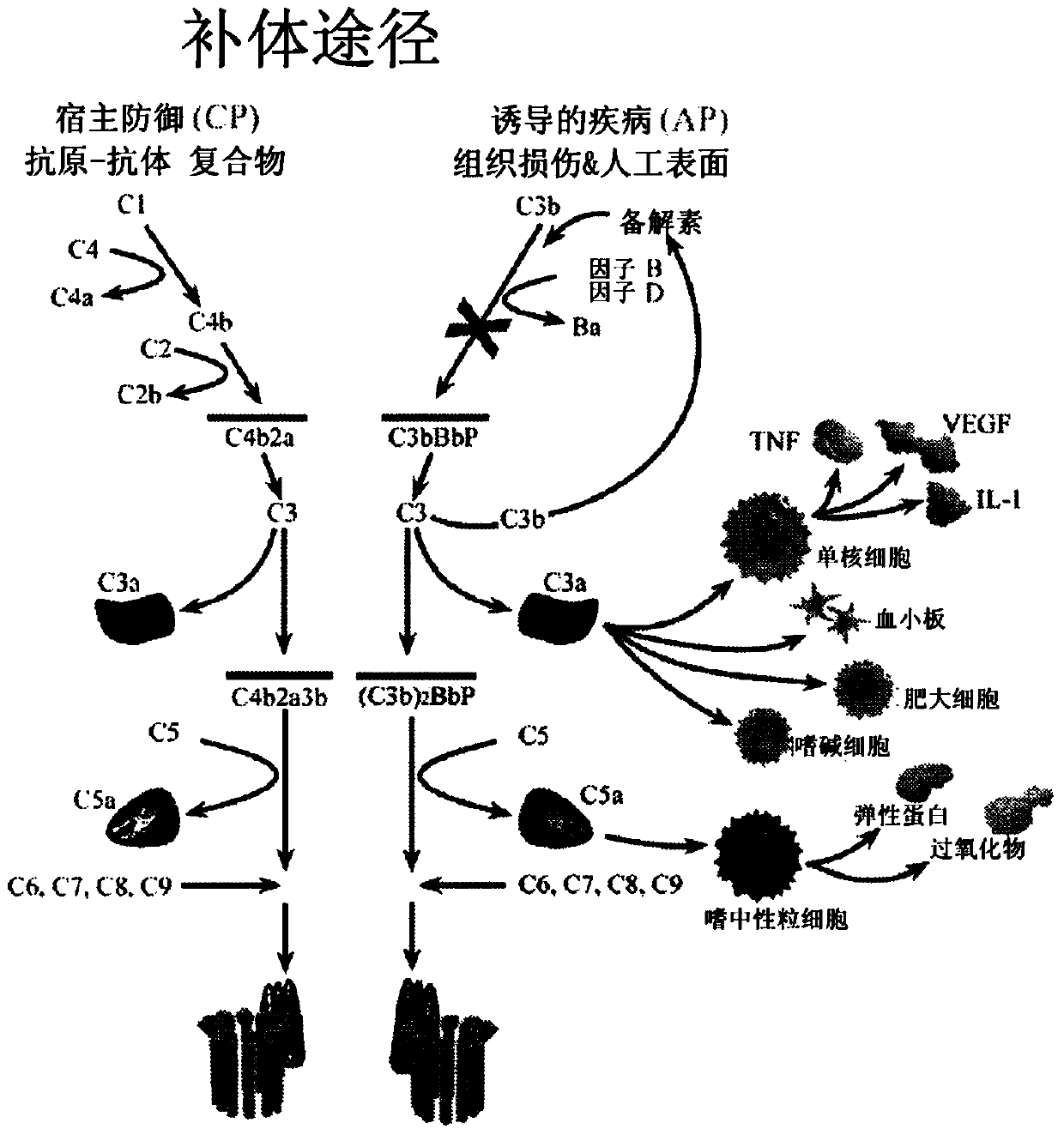
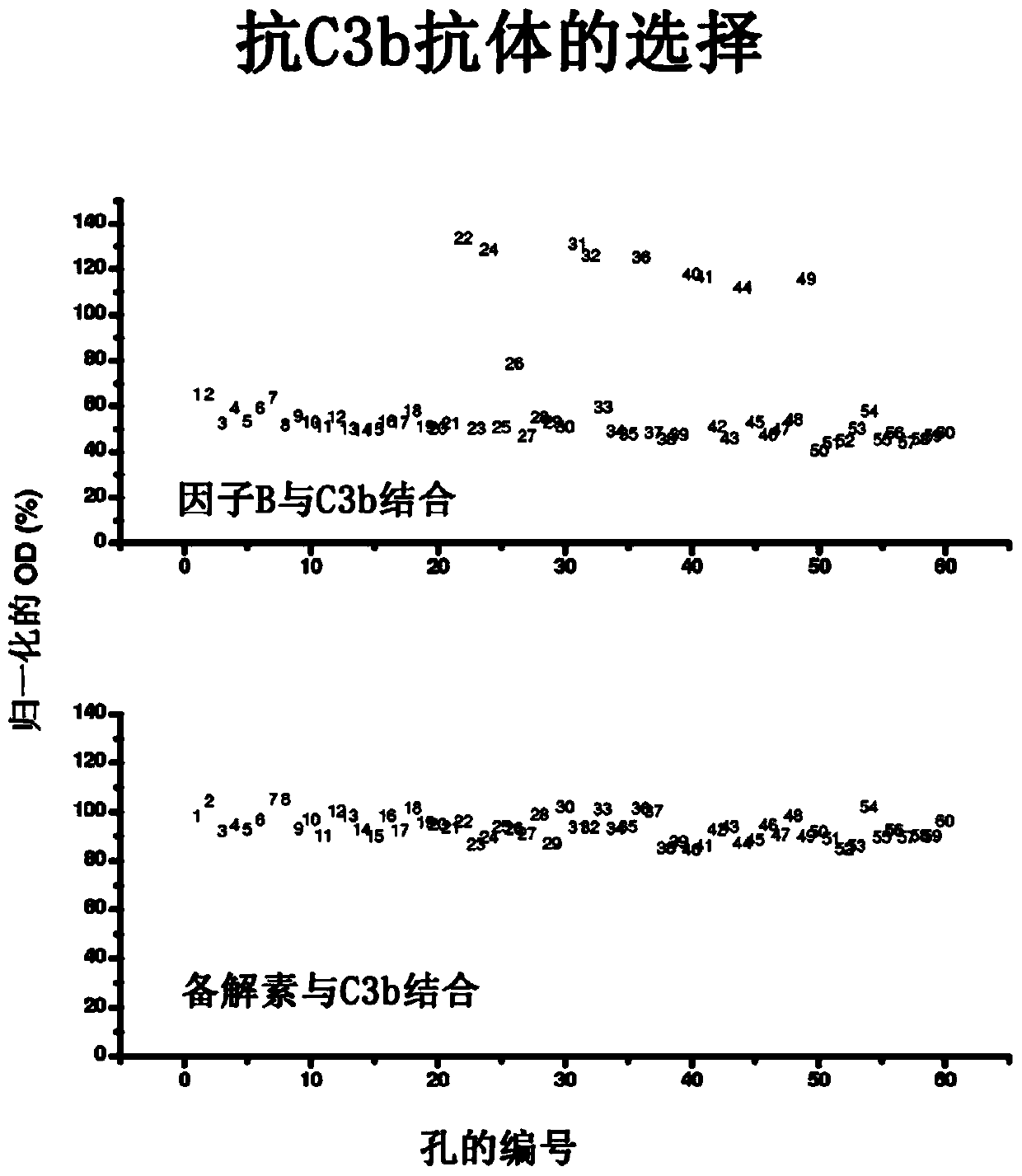
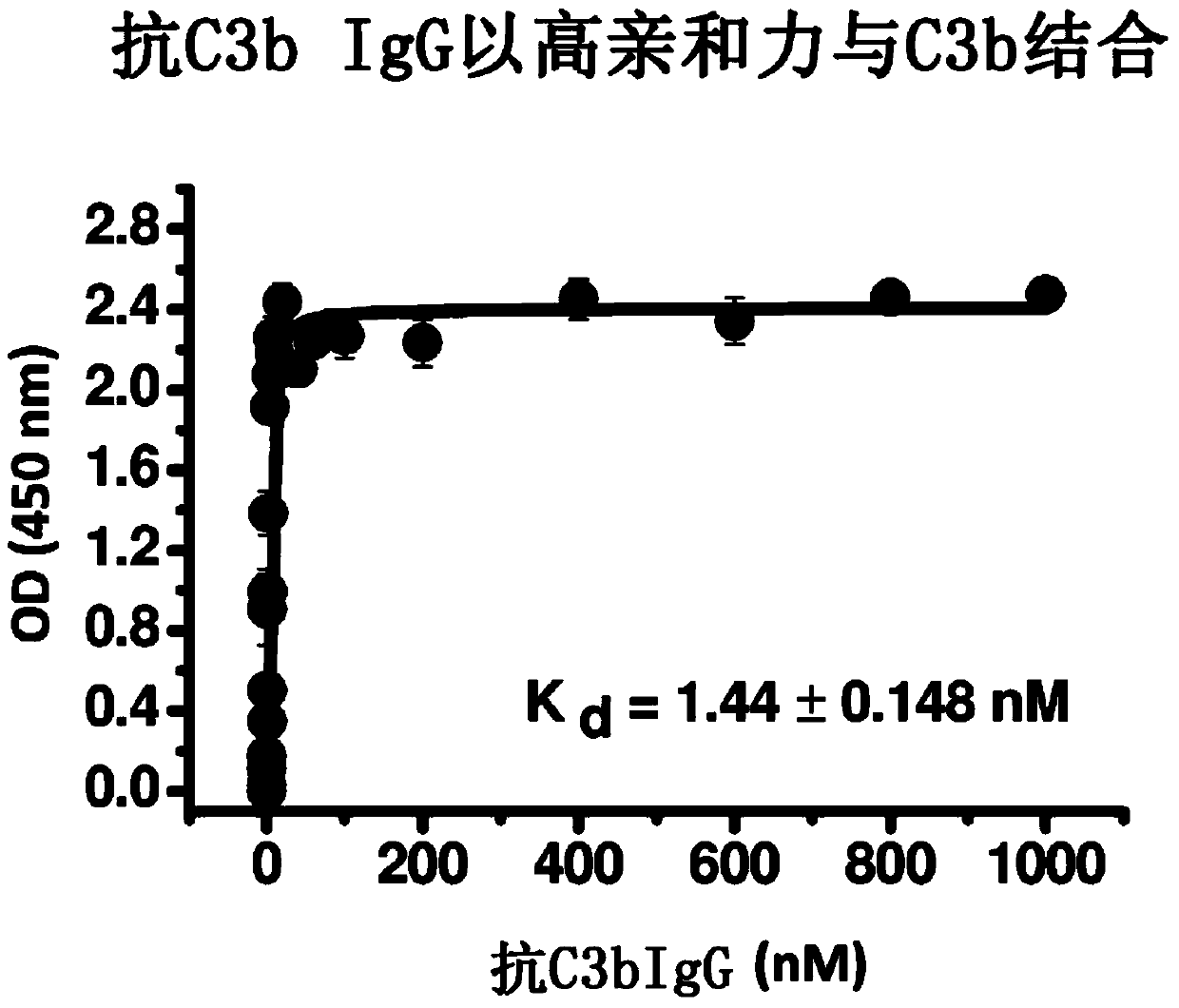
Humanized and chimeric anti-factor c3 antibodies and uses thereof
A humanized and anti-factor technology, applied in the direction of antibodies, anti-animal/human immunoglobulins, anti-inflammatory agents, etc., can solve the problem of lack of specific targeting and inhibition for human use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] All reagents were used in high quality unless otherwise stated. All complement proteins, alternative and classical pathway buffers, detection antibodies, and red blood cells were obtained from Complement Technologies (Tyler, TX) or Quidel Corporation (San Diego, CA). Flow cytometry antibodies were obtained from BD Biosciences, San Jose, CA. TMB substrate was obtained from Kirkegaard & Perry Limited, Gaithersberg, MD. All secondary antibodies were obtained from American Qualex, San Clemente, CA, BSA and other reagents were obtained from Sigma-Aldrich, St Louise, MO.
[0095] ELISA plate readers (SpectraMax 190 and 250) were obtained from Molecular Devices and the flow cytometer was a FACS Calibur. The Varity 3D program was used for data analysis. Curve fitting was performed using the MicroCal Origin program. Kinetic tests for hemolysis were run using SectraMax, Molecular Devices. ELISA plates were obtained from Corning Costar, Lowell, MA.
[0096]The humanized chim...
Embodiment 2
[0100] Example 2: Anti-C3b IgG does not inhibit the interaction of properdin C3b
[0101] Anti-C3b IgG has a low pM to low nM affinity for C3b. Antibodies and fragments thereof bind C3b and C3c with high affinity. These antibodies and fragments thereof do not inhibit the binding of properdin to C5. Polystyrene microtiter plates were coated with human factor C3b in phosphate buffered saline (PBS) overnight at 4°C. After aspiration of the C3b solution, wells were blocked with 1% bovine serum albumin (BSA) in PBS (Sigma-Aldrich, St. Louis, Mo.) for 1 hour at room temperature. Wells with no peptide or C3b coating served as background controls. Aliquots of monoclonal anti-C3b antibodies IgG, Fab2, and Fab in blocking solution (containing 2 nM biotinylated properdin) were added to C3b-coated wells and incubated for 1 hour to allow binding to occur . After an incubation period of 1 hour at room temperature, the plates were washed 5 times with PBS and incubated with peroxidas...
Embodiment 3
[0103] Example 3: Anti-C3b IgG, F(ab')2, and Fab inhibit alternative pathway (AP)-dependent rabbit red Cell (rRBC) lysis
[0104] The present test for erythrolysis is based on the formation of terminal complement complexes on the rRBC surface. As a result, the rRBCs dissolve. The programmed decrease in light dispersion at 900 nm is a direct measure of erythrocyte lysis. Typically, rRBCs contain 5mM MgCl 2 The gel was incubated with normal human serum in barbiturate buffer. Under these conditions, the surface of rRBCs triggers activation of the alternative pathway in normal human serum. Activation of the alternative pathway leads to the formation of the C5b-9 complex on the surface of rRBCs. Agents that inhibit C5b-9 complex formation are expected to inhibit cell lysis. In order to evaluate the effect of anti-C3b antibody and its fragments, various concentrations of IgG, F(ab') 2 and Fab were incubated with normal human serum (10% NHS) in AP buffer with a fixed conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com