Amorphous calcium bucladesine sterile powder
A technology of calcium dibutyryl cyclic adenosine monophosphate and sterile powder, which is applied in the field of preparation of amorphous dibutyryl cyclic adenosine calcium monophosphate sterile powder, can solve problems such as allergic reactions and abnormal vision, and achieve high separation, impurity types and The effect of content reduction, purity and stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
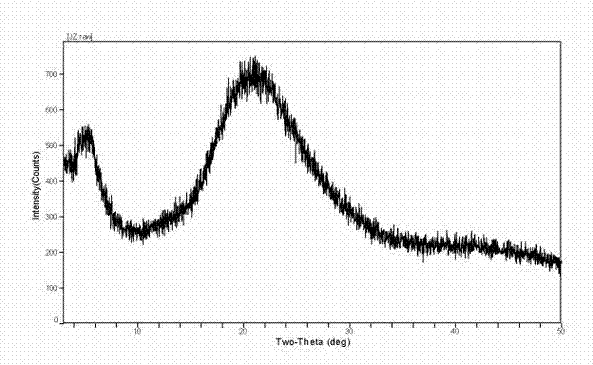
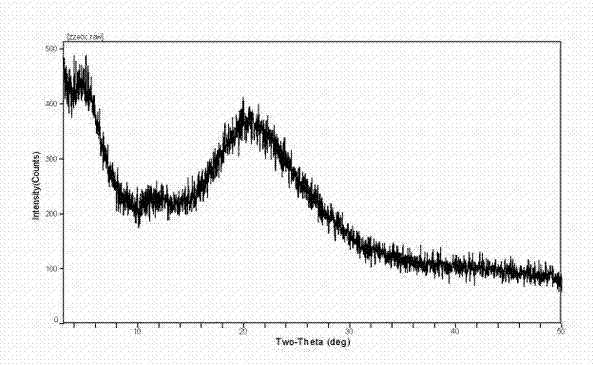
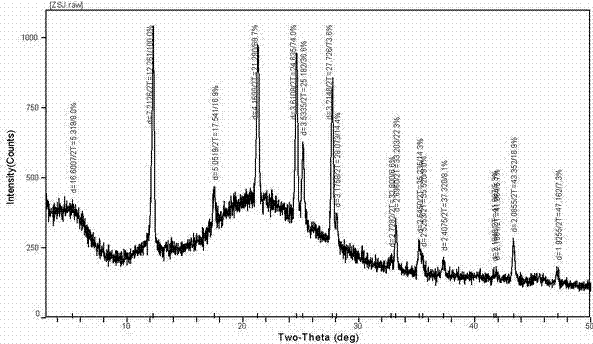
[0040] Preparation of Sterile Powder of Amorphous Calcium Dibutyryl Cycloadenosine Phosphate
[0041] (1) Add triethylamine water (1:5, v / v) solution to 200.0g cyclic adenosine monophosphate, stir at 30-40°C, evaporate to dryness under reduced pressure, add 1000ml pyridine, repeat once, vacuum decompression Dry to obtain intermediate 1, and detect the content of the intermediate.
[0042] (2) Take 250.0 g of the above intermediate 1 and add 10-15 times the volume of pyridine and 5-10 times the volume of n-butyric anhydride for acylation, and stir at 30-35 ° C. Absorb the acid anhydride with an appropriate amount of diethyl ether at low temperature, extract with 2-3 times the volume of water, repeat two to three times, and concentrate the obtained dibutyrylcyclic adenosine triethylamine saline solution under reduced pressure to dryness to obtain intermediate 2;
[0043] (3) The above intermediate 2 was dissolved with 3-5 times (m / v) ethanol solution, and an equivalent amount o...
Embodiment 2
[0049] Example 2 Amorphous calcium dibutyryl cyclic adenosine monophosphate sterile powder preparation
[0050] Dibutyryl cyclic adenosine monophosphate calcium aseptic powder prepared in the above example is divided into vials in a sterile environment, 20 mg / bottle.
[0051] According to the Chinese Pharmacopoeia 2010 edition second appendix I B inspection items for injection of sterile powder inspection items, three consecutive batches of products were inspected. The inspection indicators are visible foreign matter, insoluble particles, sterility test, and bacterial endotoxin, all of which meet the requirements of the Pharmacopoeia.
Embodiment 3
[0052] Example 3 Amorphous calcium dibutyryl cyclic adenosine monophosphate sterile powder preparation
[0053] The amorphous calcium dibutyryl cyclic adenosine monophosphate sterile powder prepared in Example 1 is for subsequent use;
[0054] Glycine refining: first dissolve crude glycine in hot water, add powdered activated carbon for adsorption and decolorization, and then separate the activated carbon by filtration;
[0055] Under a sterile environment, the above solutions are passed through 0.45 μm and 0.22 μm microporous membranes respectively, and the filtrate is heated and concentrated. When the volume of the concentrated solution is 1 / 2 of the original volume, cool to room temperature, and then add 3-4 times the volume of non-toxic Glycine is crystallized and precipitated with bacterial ethanol or methanol, and dried to obtain sterile refined glycine;
[0056] Under a sterile environment, mix amorphous calcium dibutyryl cyclic adenosine monophosphate and glycine ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com