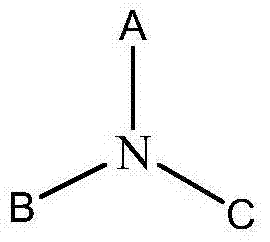
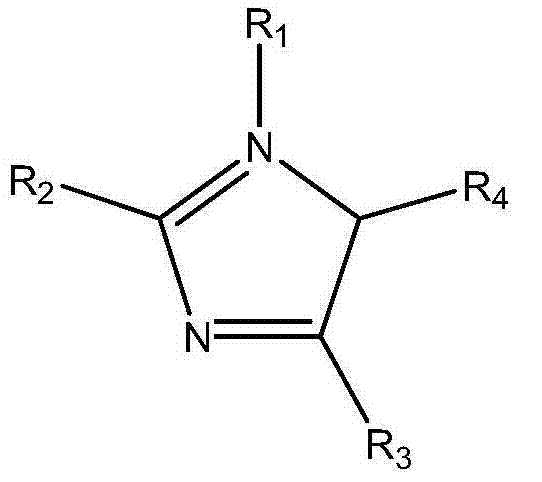
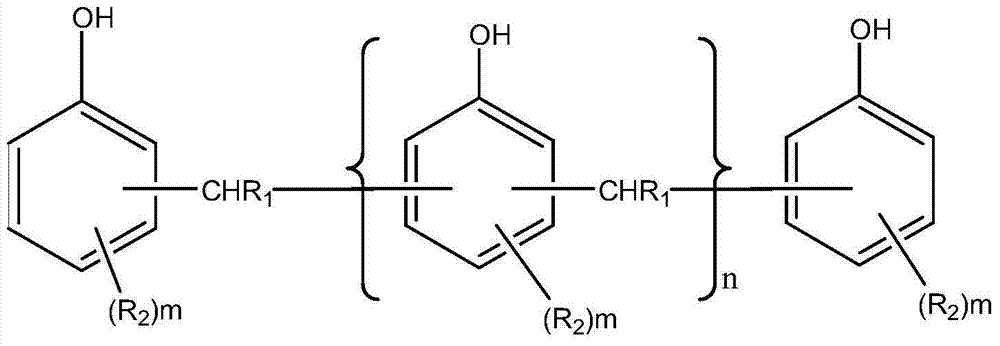
Amines and polymeric phenols and usage thereof as curing agents in one component epoxy resin compositions
一种组合物、酚树脂的技术,应用在固化剂和促进剂领域,能够解决高使用水平、低固化温度等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Preparation of Amine Solution in Polyphenol Resin
[0072] in N 2 A two-piece 500 mL glass flask was charged with the amine under atmosphere and heated to 145-180 °C with a heating mantle. Slowly add the phenolic resin (PN-320) while stirring with a mechanical stirrer. After the addition was complete the mixture was maintained at 180°C for a further period of 1 hour. Pour this molten solution into on a block or aluminum sheet and allow to cool to room temperature. The cooled solid product is ground to a particle size of about 2 to about 50 μm by a coffee grinder or jet mill. Use this method to prepare the following amine solutions.
[0073] (a) 1,3-aminopropylimidazole solution in polyphenol resin (PN320)
[0074] Amine / polyphenol resins in 180 / 250, 180 / 500 and 180 / 750 weight ratios were prepared as follows:
[0075] Using (i) 37.18g 1,3-aminopropylimidazole and 103.69g polyphenol resin (PN320), (ii) 38.39g 1,3-aminopropylimidazole and 53.50g polyphen...
Embodiment 2
[0093] Example 2: Differential Scanning Calorimetry (DSC) of Amine Solutions in Polyphenol Resin
[0094] (A) The only curing agent
[0095] A sample of the amine solution prepared according to Example 1 was thoroughly mixed with bisphenol A diglycidyl ether (10:100 mass ratio) and analyzed by DSC (TA instruments QA20) to determine the initial curing temperature, heat of reaction (ΔH) and glass transition temperature (Tg). The DSC is run according to standard methods using the software contained in the DSC. The results are shown in the table below:
[0096]
[0097] (B) Amine / phenol solid solution as a DICY accelerator
[0098] A sample of the amine solution of Example 1 was mixed with dicyandiamide (DICY) and bisphenol A diglycidyl ether (2:6:100 mass ratio) in a 250 ml glass flask by using a mechanical stirrer. The mixture was analyzed by DSC in the manner described above to determine the onset temperature of cure, heat of reaction (ΔH) and glass transition temperatur...
Embodiment 3
[0100] Example 3: Latency of a solution of amine in polyphenol resin (PN320) in epoxy resin
[0101] The latency of the amine / polyphenol resin solution prepared according to Example 1 was monitored by a Brookfield cone and plate viscometer (Model HADV II+CP) with #52 spindle at 25°C using a 0.5 mL sample. Storage stability was also determined by visual observation to determine the gel time. The results are shown in the table below:
[0102] Sole curing agent (amine / bisphenol A diglycidyl ether (10 / 100))
[0103]
[0104]
[0105] Amine / DICY / Bisphenol A diglycidyl ether (2 / 6 / 100)
[0106]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| lap shear strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com