Rebamipide monohydrate crystal form and preparation method thereof
A technology of monohydrate and rebamipide, which is applied in the field of crystal form of rebamipide monohydrate and its preparation, can solve the problems affecting the stability, dissolution and bioavailability of preparations, affecting the quality and safety of pharmaceutical preparations issues of sexuality and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of rebamipide sodium salt or potassium salt
[0038] Add 600ml of methanol, 64g of rebamipide, 17g of sodium hydroxide or 18g of potassium hydroxide into a 1000ml three-necked flask, stir and heat up to 60-65°C, the solid is completely dissolved, filter while it is hot, and cool the filtrate to 0-5°C for analysis. crystallize for 3 hours, filter with suction, wash the filter cake with ice methanol, dry under reduced pressure at 60°C for 6-7 hours, get 64g of rebamipide sodium salt or 66g of rebamipide potassium salt.
Embodiment 2
[0039] Example 2 Preparation of rebamipide monohydrate crystal form
[0040] Add 30ml of methanol, 12ml of water, and 3g of rebamipide sodium salt prepared according to Example 1 into a 100ml three-necked flask, heat to 60-80°C to completely dissolve the solid, add 0.03g of activated carbon, and reflux for 1h for decolorization. Filtrate hot, cool the filtrate to room temperature 20-25°C, adjust to 2-5°C with concentrated hydrochloric acid, precipitate a solid, stir for 1 hour, filter with suction, wash the filter cake with 30ml of ice water, and dry it in vacuum at 40°C for 15 hours to obtain the Ruixin of the present invention. Bappet monohydrate crystal form.
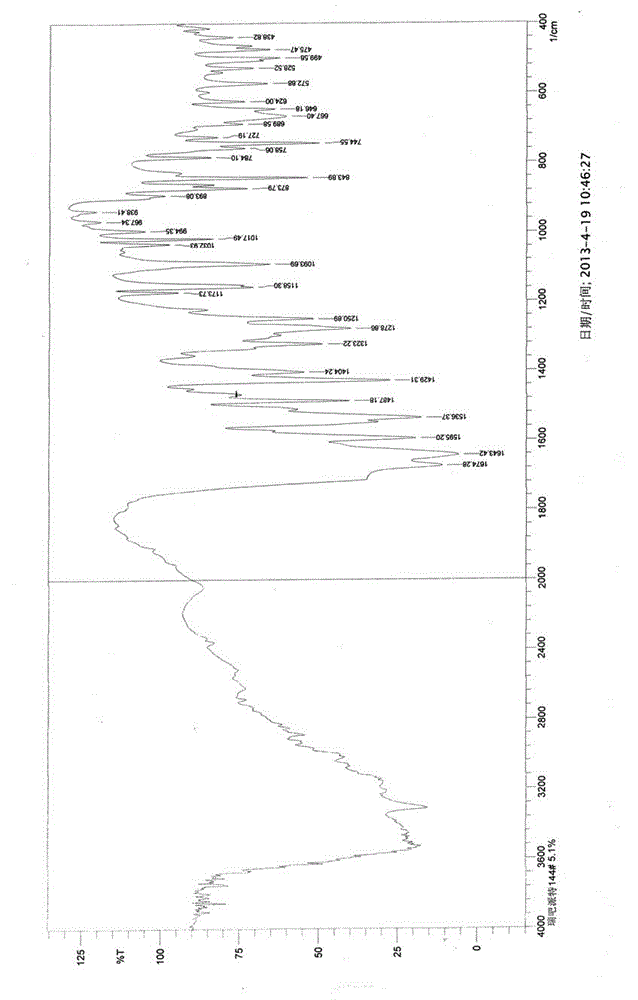
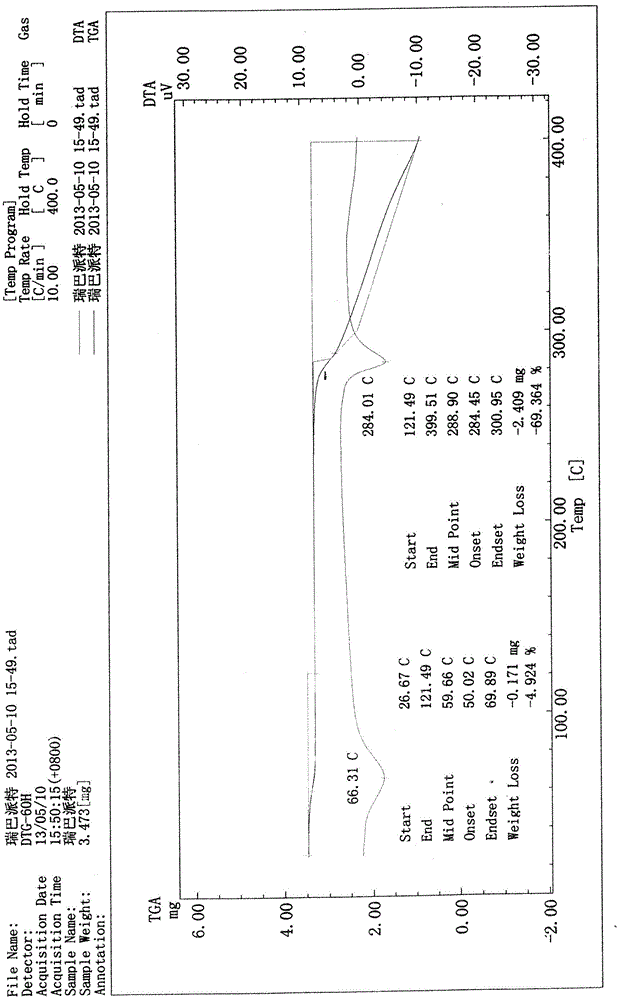
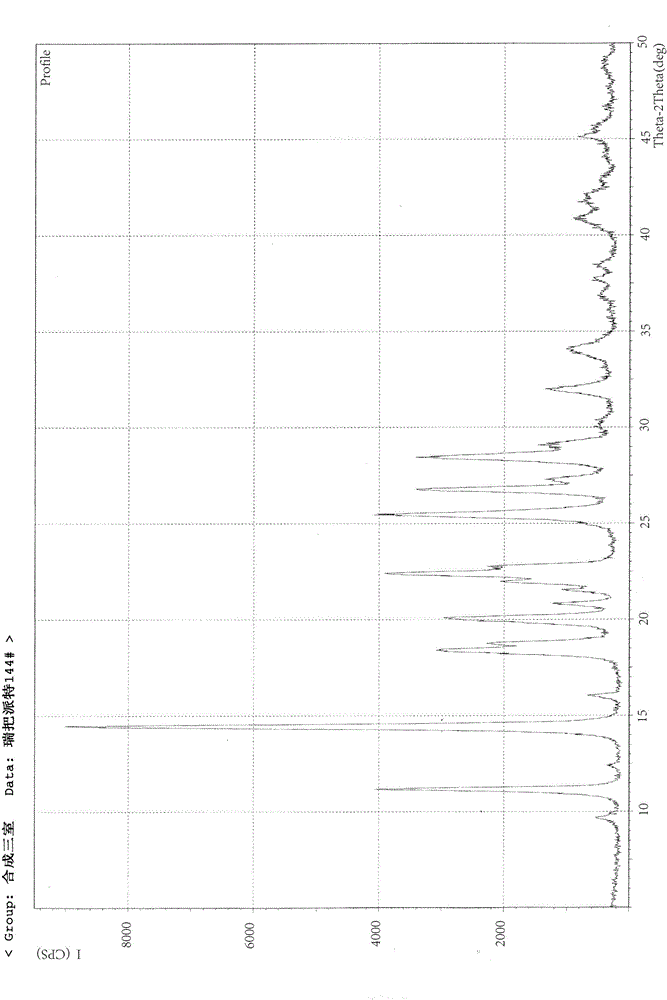
[0041] X-ray powder diffraction pattern (XRPD) see attached figure 1 ; Infrared absorption spectrum (IR) see attached figure 2 ; Differential thermal and thermal gravimetric analysis test chart (DTA-TG) see attached image 3 ; Loss on drying: 4.96% (test method: 105°C, 3h, drying under normal pressure).
Embodiment 3
[0042] Example 3 Preparation of rebamipide monohydrate crystal form
[0043] Add 60ml of ethanol, 30ml of water, and 3g of rebamipide potassium salt prepared according to Example 1 into a 100ml three-necked flask, heat to 60-80°C to completely dissolve the solid, add 0.03g of activated carbon, and reflux for 1h for decolorization. Filtrate hot, cool the filtrate to room temperature 20-25°C, adjust to 2-5°C with concentrated hydrochloric acid, precipitate a solid, stir for 1 hour, filter with suction, wash the filter cake with 30ml of ice water, and dry it in vacuum at 40°C for 15 hours to obtain the Ruixin of the present invention. Bappet monohydrate crystal form.
[0044] The X-ray powder diffraction spectrum, infrared absorption spectrum, differential thermal and thermogravimetric analysis test spectrum are consistent with the crystal form obtained in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com