Bosutinib compound
A technology for bosutinib and a composition, which is applied to bosutinib monohydrate and its preparation, and the application field of medicine, can solve the problems of difficult to effectively improve product purity, difficult to achieve, poor quality reproducibility, etc.
Inactive Publication Date: 2015-03-25
TIANJIN HANKANG PHARMA BIOTECH
View PDF3 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
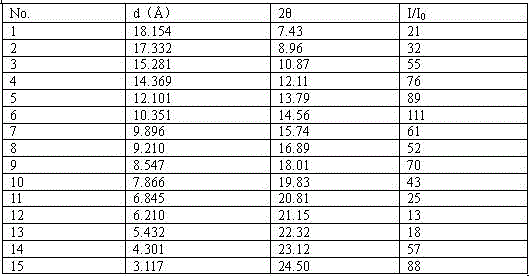
However, the monohydrate crystal form obtained above has the following problems in the production process: (1) the yield is low, and it is difficult to realize in industrial production; (2) it is difficult to effectively improve the product purity, and it is difficult for a single impurity to reach 0.1% Below, and poor quality reproducibility during repetition
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
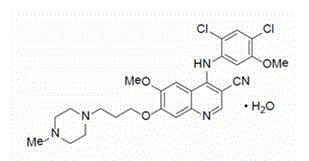
Belonging to the technical field of medicine, the invention in particular relates to a bosutinib monohydrate and a preparation method thereof. The bosutinib monohydrate obtained by the invention contains a crystal water, and has the advantages of: good stability, easy industrialized preparation process, good quality reproducibility, and well controlled product purity. The invention also relates to application of compositions using the hydrate in drugs treating chronic myeloid leukemia.
Description
technical field [0001] The invention belongs to the technical field of medicine, and in particular relates to bosutinib monohydrate and a preparation method thereof. The present invention also relates to the application of the hydrate composition in medicine for treating chronic myelogenous leukemia. Background technique [0002] Bosutinib is a potent protein kinase (Src-Abl) inhibitor, which can not only inhibit the autophosphorylation of Src protein in a variety of human tumor cells, but also inhibit the phosphorylation of Src and Abl substrates process. The drug was developed by Wyeth Pharmaceuticals, a subsidiary of Pfizer in the United States, and was first launched in the United States in September 2012. It is approved for chronic phase, accelerated phase or blast phase that is resistant or intolerant to previous treatment The treatment of adult patients with Philadelphia chromosome-positive chronic myelogenous leukemia (CML), the product name is Bosulif, and the spe...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D215/54A61K31/496A61P35/02
CPCC07D215/54
Inventor 严洁李轩
Owner TIANJIN HANKANG PHARMA BIOTECH
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com