Method for drug release by cleavage of ester bond
A technology of drugs and ester bonds, applied in the field of drug release, can solve the problems of slow sulfhydryl exchange process and changing drug function, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
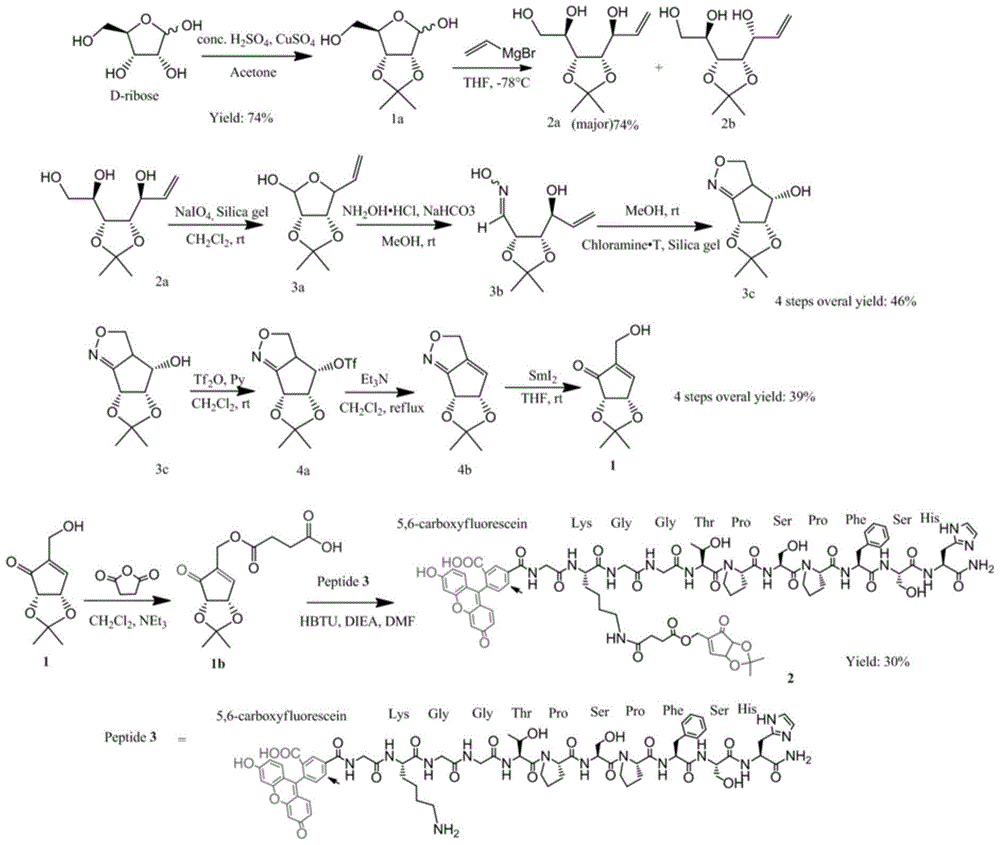
[0029] Such as figure 1 As shown, the drug small molecule Preparation: (1) D-ribose (80g, 532.9mmol) was dissolved in acetone 1000ml, and H was added dropwise at room temperature 2 SO 4 (2.4ml) and stirred at room temperature for 2.5 hours, the mixture was washed with solid NaHCO 3 Neutralized, filtered and concentrated by evaporation under reduced pressure to give a colorless syrup (1a), yield 74%.
[0030] (2) Compound 1a (20.36g, 107.1mmol) was dissolved in anhydrous THF (800ml), and vinylmagnesium bromide (480.0ml, 480.0mmol, 1.0M THF solution) was added dropwise at -78°C, and the reaction mixture was Stirring at 0°C for 3 hours afforded the compound (2a), the content of compound 2a (main product) in the product (2a+2b) is 74%.
[0031] (3) Compound 2a (19.25g, 88.2mmol) was used in CH 2 Cl 2 (330ml) solution, sodium periodate (204.0ml, 132.4mmol, 0.65M aqueous solution) solution was added dropwise at 0°C, and the reaction mixture was stirred at room temperature...
Embodiment 2
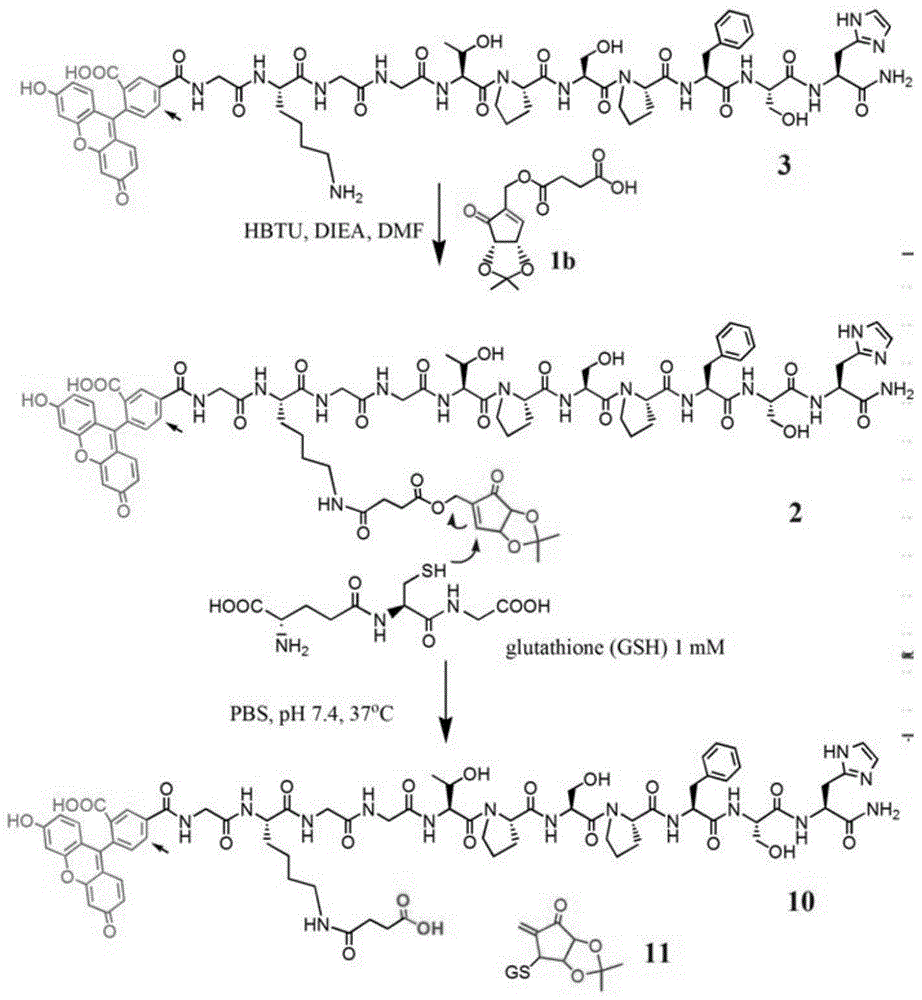
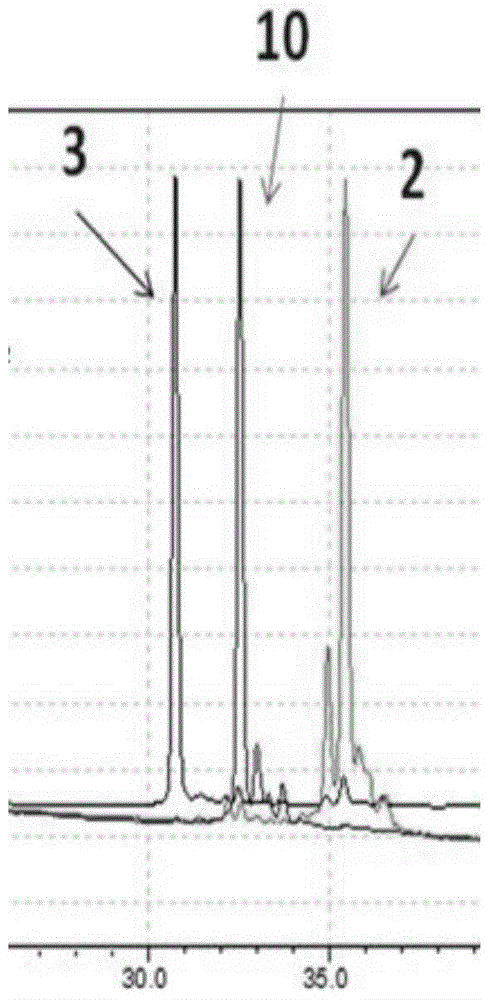
[0042] Change the short peptide sequence TPSPFSH to RGD, and the rest of the steps are the same as in Example 1. The addition reaction of glutathione and RGD polypeptide-small molecules leads to the cleavage of ester bonds for drug release.
Embodiment 3
[0044] The drug small molecule 1b was changed to doxorubicin, and the rest of the steps were the same as in Example 1. The addition reaction of glutathione and TPSPFSH polypeptide-doxorubicin results in the breakage of the ester bond for drug release.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com