Azadibenzocyclooctine compounds and preparation methods thereof
A technology of benzocyclooctyne and benzocyclooctyne hydrochloride, which is applied in the field of organic chemical synthesis, can solve problems that have not been reported, and achieve the effects of easy to obtain raw materials, simple raw materials, convenient post-treatment and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
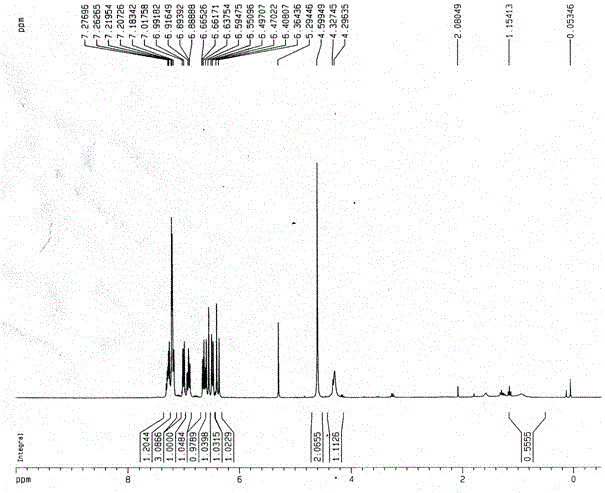
[0058] Synthesis of the following compounds
[0059]
[0060] Add 40g of the compound 5-dibenzocycloheptenone represented by formula (II), 67.4g of hydroxylamine hydrochloride into a 1L round bottom flask, then add 480mL of absolute ethanol, 104mL of pyridine, heat and reflux for 15h; monitor the reaction by TLC After completion, cool to room temperature, dilute the system with 100mL ethyl acetate, add 500mL 1M hydrochloric acid, stir for half an hour; ), the organic phase was dried with anhydrous sodium sulfate, filtered, and concentrated to obtain 41.3 g of the above compound as a white solid, with a yield of 96%.
Embodiment 2
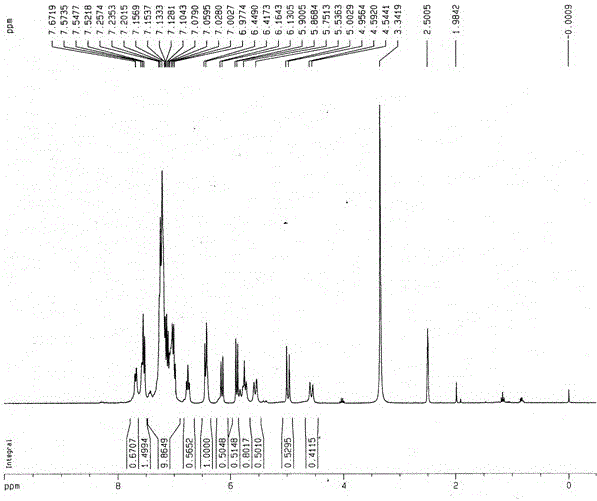
[0062] Synthesis of the following compounds
[0063]
[0064] Add 48g of compound 3,7-difluoro-5-dibenzocycloheptenone and 68g of hydroxylamine hydrochloride to a 1L round-bottomed flask, then add 500mL of absolute ethanol and 104mL of pyridine, heat and reflux for 15h; monitor the reaction by TLC After completion, cool to room temperature, dilute the system with 100mL ethyl acetate, add 500mL 1M hydrochloric acid, stir for half an hour; ), the organic phase was dried with anhydrous sodium sulfate, filtered, and concentrated to obtain 49.1 g of the above compound as a white solid, with a yield of 95%.
Embodiment 3
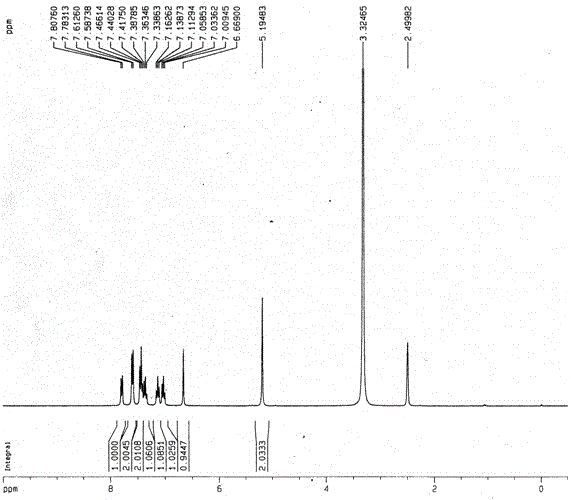
[0066] Synthesis of the following compounds
[0067]
[0068] Prepare Eaton's reagent: 25g of phosphorus pentoxide is dissolved in 200mL of methanesulfonic acid.
[0069] Add 41.3 g of the compound represented by formula (IIIa) into a 500 mL round bottom flask, and add the above-mentioned Eaton reagent in one batch under the protection of argon. At this point, the system immediately turned dark red, and then it was placed at 100° C. and stirred for half an hour. The reaction solution was directly poured into 1L of ice-water mixture, and a white solid was precipitated. After filtration, the filter cake was washed with water 2 to 3 times, and dried under an infrared lamp to obtain 41.2 g of a white powdery solid, namely the above compound, with a yield of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com