Preparation method and application of enzyme-supported chitosan nanoparticle
A chitosan nanometer and nanoparticle technology is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc. Low-level problems, to achieve the effect of non-toxic components, small damage to protein molecules, and avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
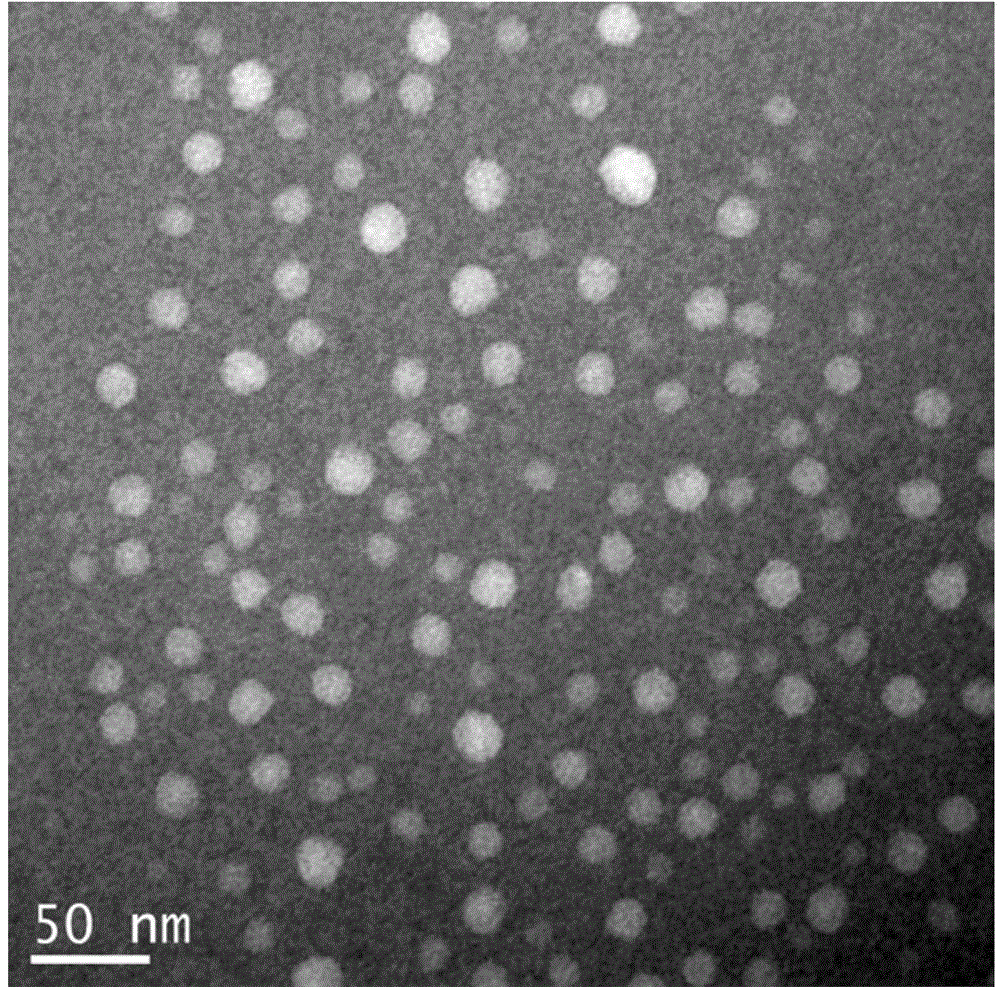
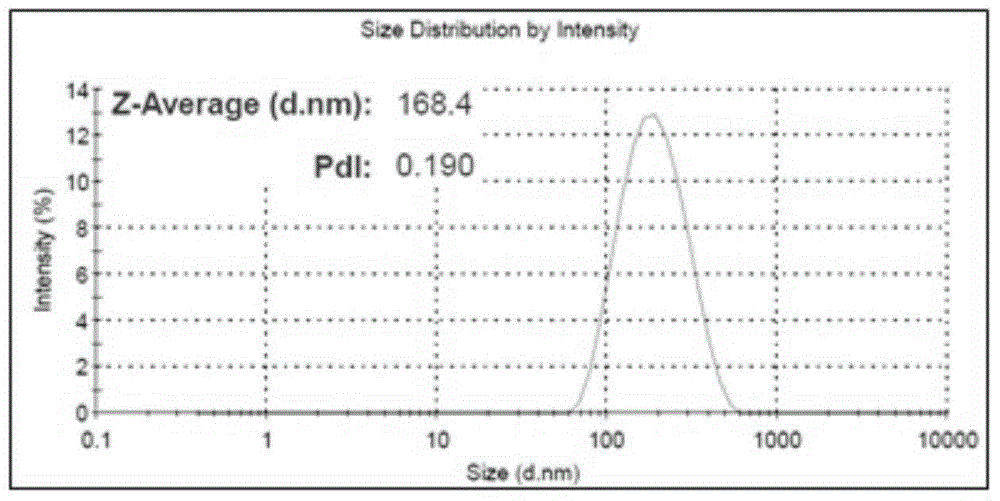
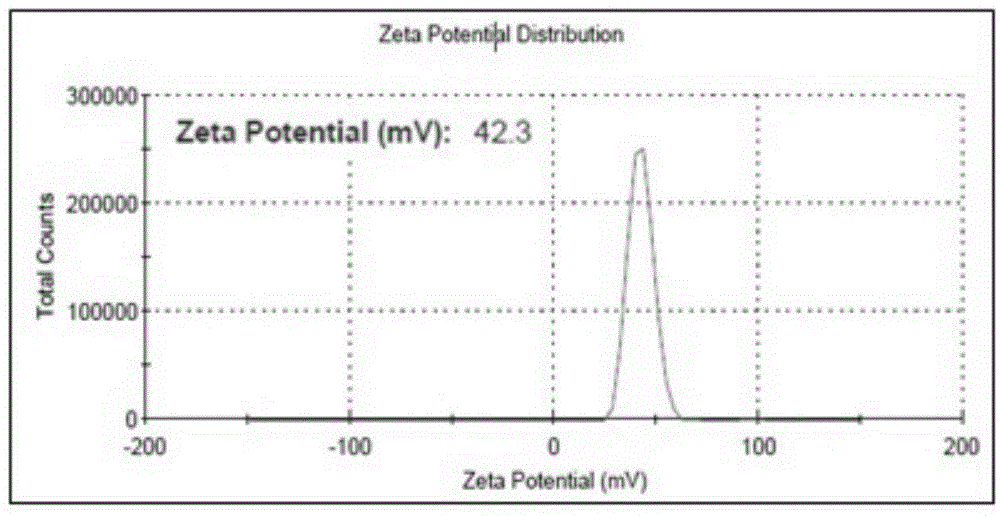
[0065] Embodiment 1. Preparation of enzyme-loaded chitosan nanoparticles (HRP-CS-NPs)
[0066] First, 0.5 mg of horseradish peroxidase (HRP) was dissolved in 1 mL of ultrapure water, and 50 mg of the chemical cross-linker 1-ethyl-3-[3-dimethylaminopropyl]-carbonated Diimine (EDC) was magnetically stirred in the above mixture for 30 minutes to activate the carboxyl groups on the surface of horseradish peroxidase (HRP). Then, 10 mg of chitosan (Chitosan) was dissolved in 1 mL of 1% acetic acid solution, and the pH was adjusted to 5.0 after magnetic stirring at 60° C. for complete dissolution. Finally, mix the 10mg / mL chitosan solution with the above-mentioned activated horseradish peroxidase (HRP) mixture, add 40mg of N-hydroxysuccinimide (NHS), and stir magnetically at 4°C for 48h To obtain the coupling complex (HRP-CS) of chitosan molecules and horseradish peroxidase (HRP).
[0067] Add 5 mL of chitosan molecule and horseradish peroxidase (HRP) coupling complex (HRP-CS) with...
Embodiment 2
[0069] Embodiment 2. The influence of different acetic acid concentrations on the preparation of chitosan nanoparticles
[0070] The chitosan of 100mg is dissolved in the acetic acid solution of 100mL (0.5%, 0.8%, 1%, 1.2%, 1.5%, 2%) respectively, is mixed with the chitosan solution of 1mg / mL, and magnetic force stirs to dissolve overnight, And regulate chitosan solution pH to 5.0 with 20wt% sodium hydroxide, and remove the insoluble impurity in the solution with the microporous membrane of 0.22 μm, make chitosan stock solution; Prepare the sodium tripolyphosphate (TPP) of 1mg / mL Aqueous solution, through a 0.22 μm microporous membrane to remove insoluble impurities in the solution to prepare TPP stock solution.
[0071] Chitosan nanoparticles (CS-NPs) preparation: 5 mL of chitosan (CS) with a pH of 5.0 at 1 mg / mL was added to a 10 mL beaker and stirred, the stirring speed was controlled to be 500 rpm, and the stirring ambient temperature was about 10 ° C. Add 1 mL of 1 mg / mL...
Embodiment 3
[0073] Embodiment 3. The impact of different chitosan concentrations on the preparation of chitosan nanoparticles
[0074] Take a certain amount of chitosan nanoparticles and dissolve them in 1% acetic acid solution, and prepare the concentrations as (0.5mg / mL, 0.8mg / mL, 1mg / mL, 1.5mg / mL, 1.8mg / mL, 2mg / mL) of the chitosan solution, magnetically stirred overnight and dissolved, and adjusted the pH of the chitosan solution to 5.0 with 20wt% sodium hydroxide, and removed the insoluble impurities in the solution with a microporous membrane of 0.22 μm to prepare the chitosan stock solution; prepare a 1 mg / mL sodium tripolyphosphate (TPP) aqueous solution, and remove insoluble impurities in the solution through a 0.22 μm microporous membrane to obtain a TPP stock solution.
[0075] Chitosan nanoparticles (CS-NPs) preparation: the concentration of (0.5mg / mL, 0.8mg / mL, 1mg / mL, 1.5mg / mL, 1.8mg / mL, 2mg / mL) shell at pH 5.0 Add 5mL of polysaccharide (CS) solution into a 10mL beaker and ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap