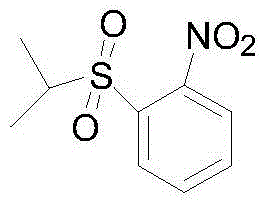
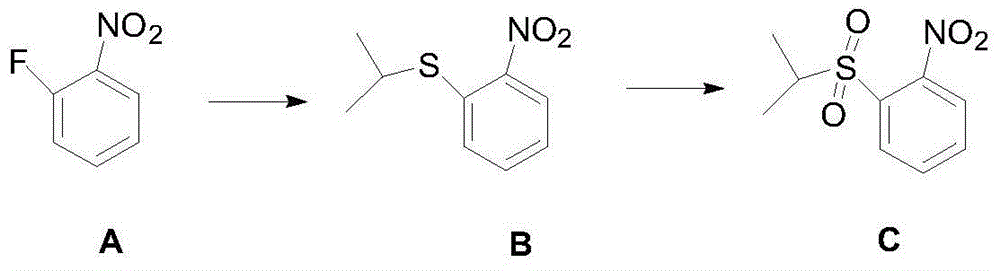
One-pot synthesis method of anticancer drug ceritinib intermediate 1-(isopropylsulfonyl)-2-nitrobenzene
A technology of propylsulfonyl and ceritinib, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as high energy consumption and long reaction time, and achieve low energy consumption and reduced production The effect of high cost and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add DMF (423.3g), 60% sodium hydride (44g, 1.1moL) and o-fluoronitrobenzene (141.1g, 1.0moL) to a reaction flask equipped with a liquid caustic tail gas absorption device, and control the temperature with an ice-water bath at 0 ℃, add isopropyl mercaptan (91.4g, 1.2moL) dropwise, the dropwise addition is completed, rise to 25℃ and react for 2.0-3.0h, the central control o-fluoronitrobenzene disappears, the reaction is completed, control the temperature at 25℃, and add dropwise Acetic acid (72.6g, 1.2moL), 30% hydrogen peroxide (340.1g, 3.0moL), after the dropwise addition, slowly raise the temperature to 80-100°C for 3.0-4.0h until 2-(isopropylsulfide)nitrobenzene disappear. Cool to 25°C, pour the reaction solution into ice water (2L), extract with dichloromethane (300mL*3), wash the organic layer with saturated sodium bicarbonate solution (300mL*3), water (300mL) successively, and dry In the organic layer, dichloromethane was distilled off under reduced pressure to ob...
Embodiment 2
[0031] Add DMF (564.4g), 60% sodium hydride (48g, 1.2moL) and o-fluoronitrobenzene (141.1g, 1.0moL) to a reaction flask equipped with a liquid caustic tail gas absorption device, and control the temperature with an ice-water bath at 0 ℃, add isopropyl mercaptan (99g, 1.3moL) dropwise, the dropwise addition is completed, rise to 25℃ and react for 2.0-3.0h, the central control o-fluoronitrobenzene disappears, the reaction is completed, control the temperature at 25℃, and then add acetic acid dropwise (78g, 1.3moL), 30% hydrogen peroxide (340.1g, 3.0moL), after the dropwise addition, slowly raise the temperature to 80-100°C for 3.0-4.0h until 2-(isopropylsulfide)nitrobenzene disappears. Cool to 25°C, pour the reaction solution into ice water (3L), extract with dichloromethane (300mL*3), wash the organic layer with saturated sodium bicarbonate solution (300mL*3), water (300mL), and dry In the organic layer, dichloromethane was distilled off under reduced pressure to obtain 189.5 g...
Embodiment 3
[0033] Add DMF (564.4g), 60% sodium hydride (60g, 1.5moL) and o-fluoronitrobenzene (141.1g, 1.0moL) to a reaction flask equipped with a liquid caustic tail gas absorption device, and control the temperature with an ice-water bath at 0 ℃, add isopropyl mercaptan (114.2g, 1.5moL) dropwise, the dropwise addition is completed, rise to 25℃ and react for 2.0-3.0h, the central control o-fluoronitrobenzene disappears, the reaction is completed, control the temperature at 25℃, and add dropwise Acetic acid (90g, 1.5moL), 30% hydrogen peroxide (453.4g, 4.0moL), after the dropwise addition is completed, slowly raise the temperature to 80-100°C for 3.0-4.0h until 2-(isopropylsulfide)nitrobenzene disappears . Cool to 25°C, pour the reaction solution into ice water (3L), extract with dichloromethane (300mL*3), wash the organic layer with saturated sodium bicarbonate solution (300mL*3), water (300mL), and dry In the organic layer, dichloromethane was distilled off under reduced pressure to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com