Stabilised proteins for immunising against staphylococcus aureus
一种蛋白、免疫原性的技术,应用在抗细菌药、含有效成分的医用配制品、过敏性疾病等方向,能够解决疫苗不一致性影响、抗原不稳定、疫苗生产复杂等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
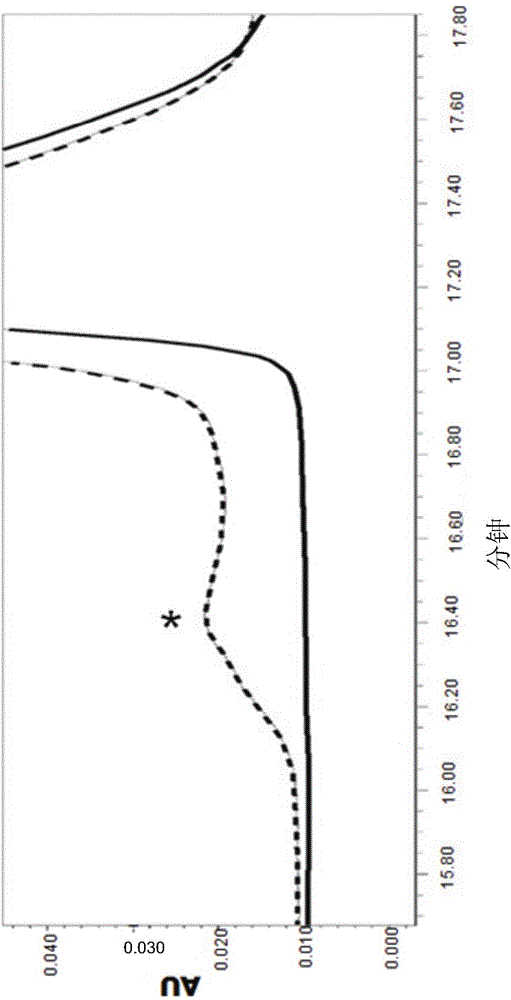
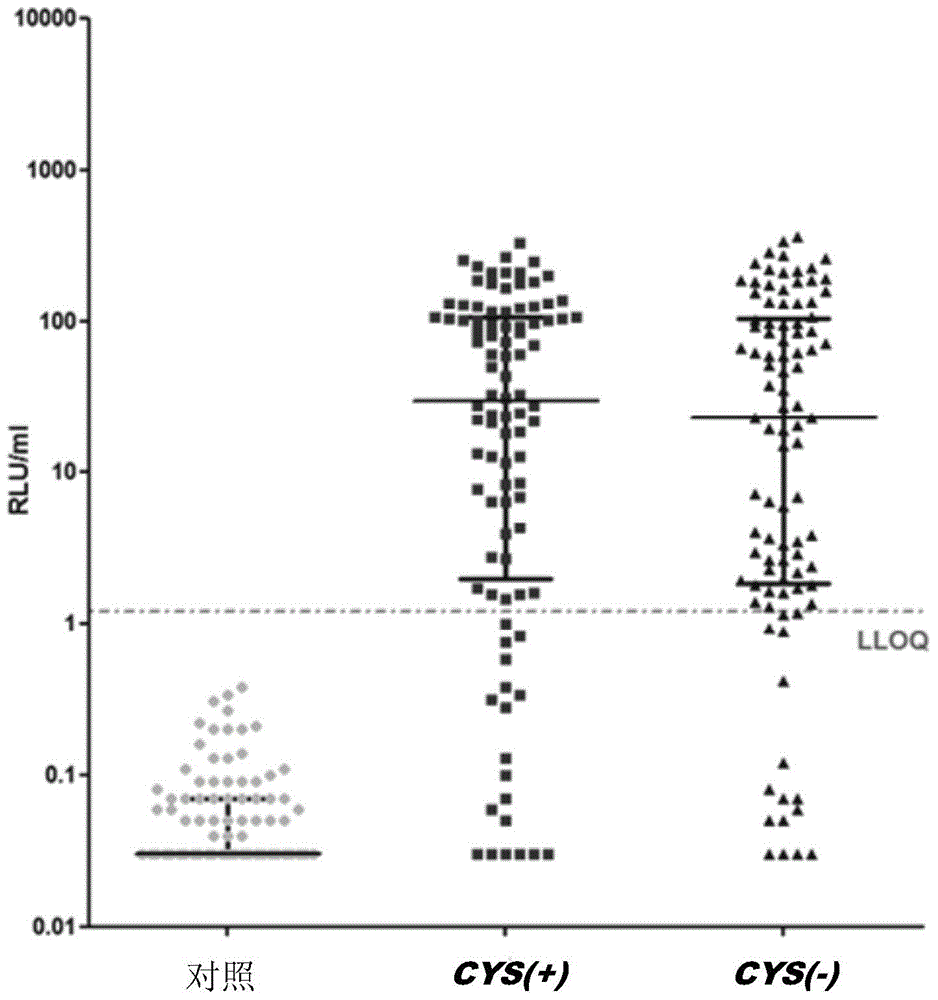
[0200] heat denaturation test
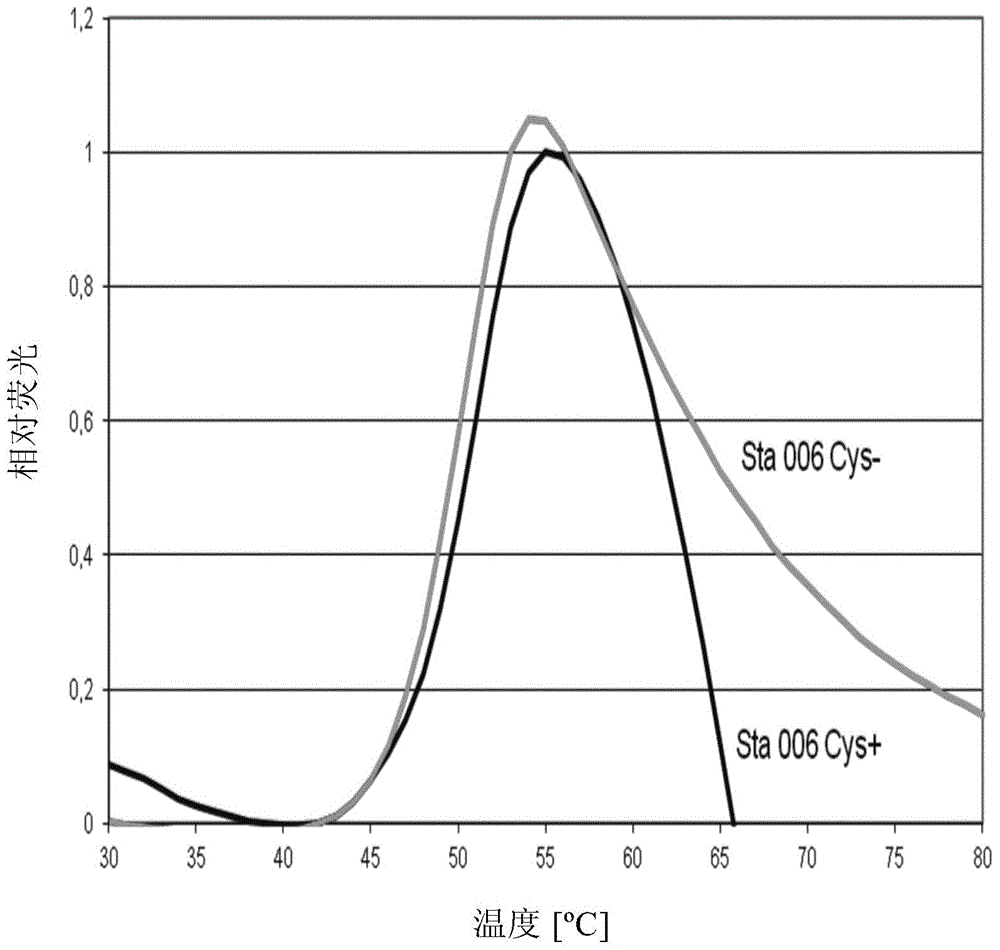
[0201] The Sta006Cys(+) antigen used in the experiments described below is represented as SEQ ID NO:3 and the Sta006Cys(-) antigen is represented as SEQ ID NO:6. Both antigens are recombinant proteins purified from E. coli.
[0202] The thermostability of Sta006 cysteine-containing Cys(+) antigen and Sta006 cysteine-deficient Cys(-) antigen was compared by differential scanning fluorescence assay (DSF). Antigen-containing samples (10 μΜ in PBS) were heated under controlled conditions in a Strategen Mx3000p real-time PCR instrument at a temperature ramp rate of 1 °C / min. The dye SyproOrange 5x was used and the change in fluorescence was monitored. The tests were carried out in the temperature range of 10-100°C.
[0203] figure 1 Melting curves for the tested antigens are reported. It shows that the peak of Cys(-) antigen is slightly shifted to the top and left compared to Cys(+) antigen. Melting temperature (Tm) was determined by fitting the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com