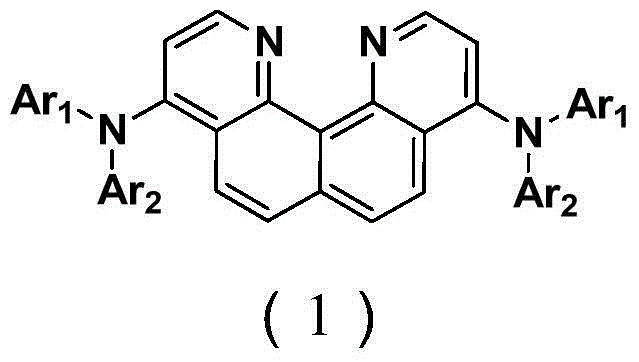
Compounded quindoline derivative and application of compounded quindoline derivative in organic electroluminescence field
A technology of quinolines and derivatives, which is applied in the application field of organic electroluminescent devices, can solve the problems of reducing device life, easy crystallization or agglomeration, and reducing device efficiency, and achieves reduced quenching and high triplet state , the effect of wide energy gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This embodiment is to prepare intermediate formula S1:
[0035] Reference (Angew.Chem.Int.Ed.Engl., 1987,26,460.) method to synthesize the 4,9-dichloroquinoline intermediate of S1.
[0036]
[0037] Its synthetic route is as follows:
[0038]
Embodiment 2
[0041] The synthesis of compound M1, the synthetic route is as follows:
[0042]
[0043] Under the protection of Ar gas, add 4.04g of diphenylamine (molecular weight 169, 0.024mol) and 40ml of anhydrous THF into a 100ml reaction flask, cool to 0°C, and slowly add 11ml of n-BuLi (2.4M, 0.026mol). Stirred at room temperature for 30min, the color turned yellow. At 0°C, take the solution for 30 minutes, slowly add S13.0g (molecular weight 299.15, 0.01mol) into 50ml THF solution, stir at 35°C for 4 hours, stir at 50°C for 8 hours, cool, and the mixture is poured into water Li, extracted with 50ml of dichloromethane, the organic phase was dried with anhydrous MgSO4, the organic phase was evaporated to dryness, and the obtained solid was separated by column chromatography to obtain 4.0 g of a yellow solid with a molecular weight of 565 and a yield of 70%.
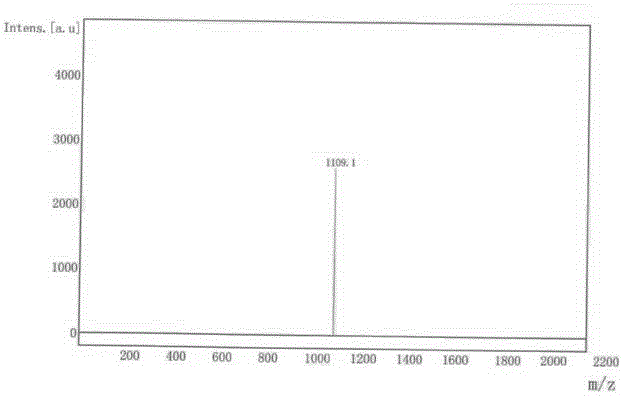
[0044]Product MS (m / e): 565, elemental analysis (C 40 h 28 N 4 ): theoretical value C: 85.08%, H: 5.00%, N: 9.92%; measu...
Embodiment 3
[0046] The synthesis of compound M2, the synthetic route is as follows:
[0047]
[0048] Under the protection of Ar gas, add 5.11g NaH (content 55%, 0.117mol) in 90ml DMF, dropwise add a solution of carbazole 16.7g (molecular weight 167, 0.1mol) dissolved in anhydrous DMF90ml, take 20 minutes, stir for 1 hour , then dissolve S113.46g (molecular weight 299.15, 0.045mol) (molecular weight 299.15, 0.045mol) in 90ml of DMF solution, add it in 20min, stir for 3 hours, pour into 500ml of water, filter the precipitate, vacuum dry, the product with silica gel After column purification, 20.2 g of the target molecule (0.036 mol) was obtained with a molecular weight of 561 and a yield of 75%.
[0049] Product MS (m / e): 561, elemental analysis (C 40 h 24 N 4 ): theoretical value C: 85.69%, H: 4.31%, N: 9.99%; measured value C: 85.60%, H: 4.34%, N: 10.05%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com