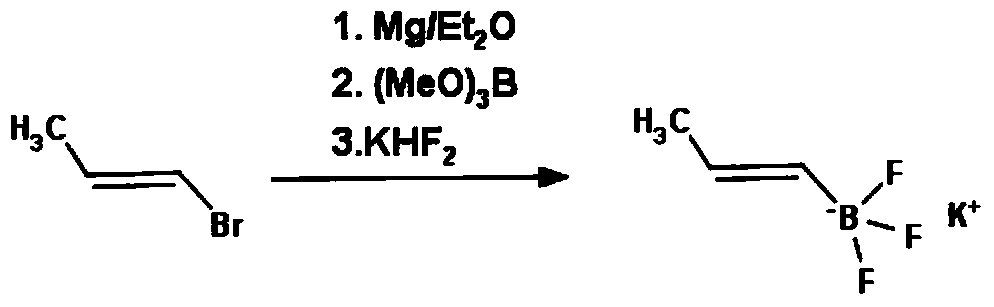
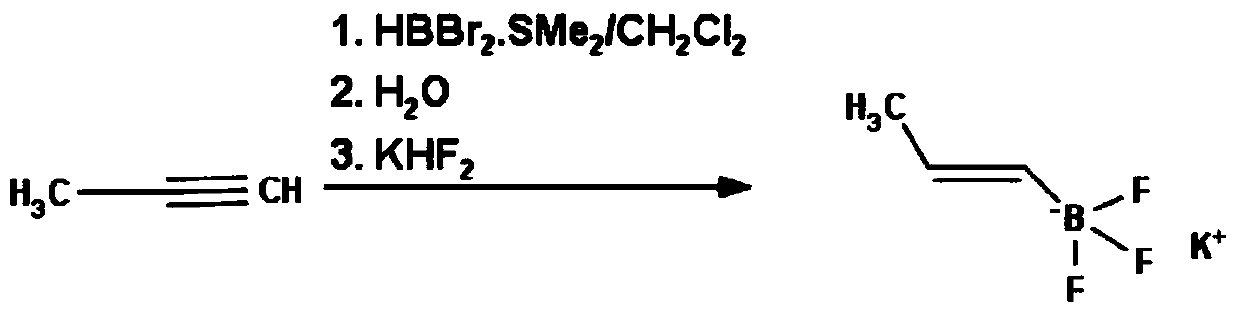
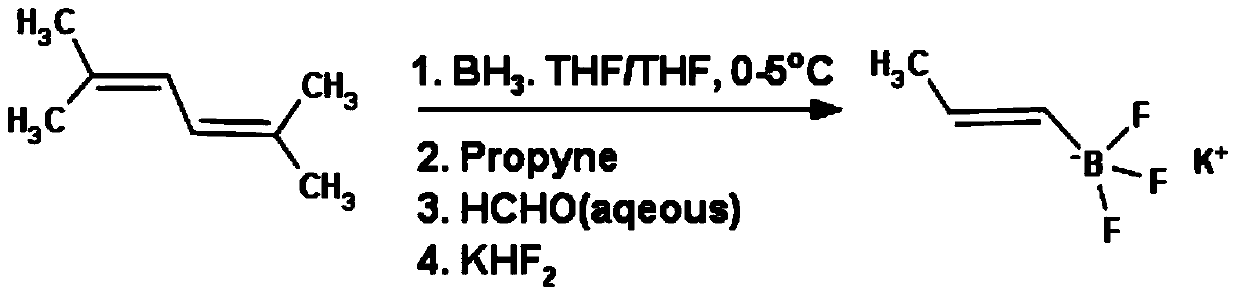
A method for preparing (e)-propene-1-potassium trifluoroborate
A technology of potassium trifluoroborate and propylene, which is applied in the field of preparation of propylene-1-potassium trifluoroborate and the synthesis of organic boric acid derivatives of pharmaceutical intermediates, which can solve the problem that the price of dibromoborane dimethyl sulfide is too expensive and the limitation Target products are produced in large quantities and are not suitable for large-scale industrial production, so as to achieve the effect of cheap raw materials, high yield and low product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] In a 1L reaction flask, add 2,5-dimethyl-2,4-hexadiene (55g, 0.5mol) and 200mL tetrahydrofuran, cool to 0°C, slowly add borane tetrahydrofuran complex (230mL, 0.22 mol, 1M in THF), after the addition, the temperature was controlled at 0-5°C, reacted for 4h, then slowly passed into propyne (8.8g, 0.22mol) at 0°C, and then rose to room temperature, reacted for 12h, cooled to 0 After ℃, slowly add water (10mL), stir for 30min, add formaldehyde aqueous solution (23g, 0.29mol, 37%), after stirring for 4h, add KHF 2 Saturated solution of (51.5g, 0.66mol), react at room temperature for 6h, evaporate the solvent under reduced pressure with a rotary evaporator, dry, extract the product with acetone, concentrate under reduced pressure, add ether to obtain 27g of white solid, which is (E)- Potassium propene-1-trifluoroborate. Yield 83%. 1H NMR (DMSO-d6): 5.57ppm, multimodal (1H); 5.21ppm, bimodal (1H); 1.53ppm, bimodal (3H).
Embodiment 2
[0029] In a 10L reaction flask, add 2,5-dimethyl-2,4-hexadiene (1102g, 10mol) and 2L tetrahydrofuran, cool to 0°C, slowly add borane tetrahydrofuran complex (4545mL, 4.55mol , 1M in THF), after the addition, the temperature was controlled at 0-5°C, reacted for 4h, then slowly passed into propyne (181g, 4.55mol) at 0°C, and then rose to room temperature, reacted for 12h, cooled to 0°C , slowly drop water (10mL), stir for 30min, add formaldehyde aqueous solution (480g, 5.92mol, 37%), after stirring for 4h, add KHF 2 The saturated solution of (1066g, 13.7mol) was reacted at room temperature for 6h, and the solvent was evaporated under reduced pressure by a rotary evaporator, then dried, the product was extracted with acetone, concentrated under reduced pressure, and ether was added to obtain 525g of a white solid, namely (E)-propylene -1-Potassium trifluoroborate, yield 78%. 1H NMR (DMSO-d6): 5.57ppm, multimodal (1H); 5.21ppm, bimodal (1H); 1.53ppm, bimodal (3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com