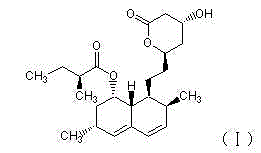
Application of lovastatin in preparation of drugs for resisting cryptosporidium parvum
A technology of Cryptosporidium parvum and lovastatin, applied in the direction of application, anti-infective drugs, drug combination, etc., to achieve good anti-Cryptosporidium effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 lovastatin powder
[0023] Preparation of lovastatin powder: take 5g of lovastatin (content not less than 99%) and 95g of starch and mix well.
[0024] Dosage of lovastatin powder: mix the above 100g powder into 100 kg of feed, and feed according to daily food intake.
Embodiment 2
[0025] Embodiment 2 lovastatin water-soluble powder
[0026] Preparation of lovastatin water-soluble powder: Take 2.5 g of lovastatin (content not less than 99%) and 97.5 g of glucose and mix well.
[0027] Dosage of lovastatin water-soluble powder: Dissolve 100g of the above-mentioned powder in 100 liters of water, and feed according to the normal amount of drinking water.
Embodiment 3
[0028] Example 3 The Anti-Cryptosporidium Parvum Effect of Lovastatin
[0029] Experimental Materials:
[0030] Control drug: Paromomycin (98%, Shanghai Fusheng Biotechnology Co., Ltd., batch number 001010) reported in the literature with anti-Cryptosporidium parvum effect, the dosage is 5 mg / kg body weight.
[0031] Lovastatin: the lovastatin powder of embodiment 1, the lovastatin water-soluble powder of embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com