Stabilised proteins for immunising against Staphylococcus aureus
An immunogenic, carrier protein technology, applied in peptide/protein components, vaccines, organic chemistry, etc., which can solve the problems of complex vaccine production, requirements affecting quality and regulatory approval, and unstable antigens.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
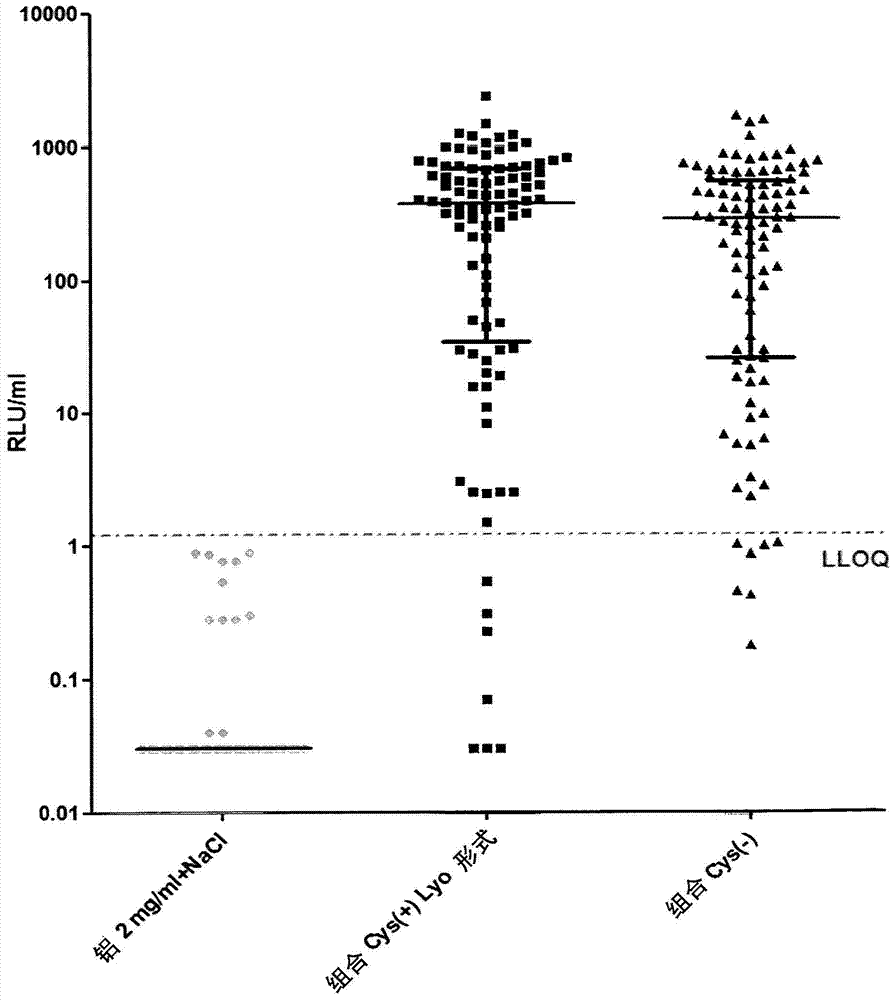
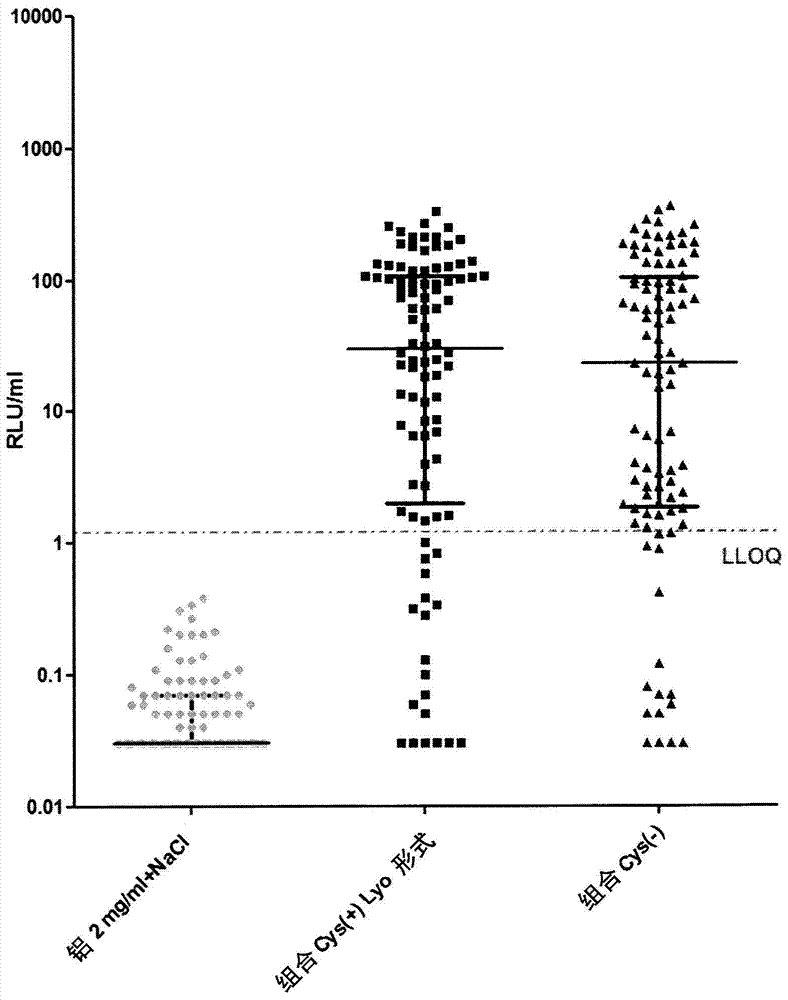
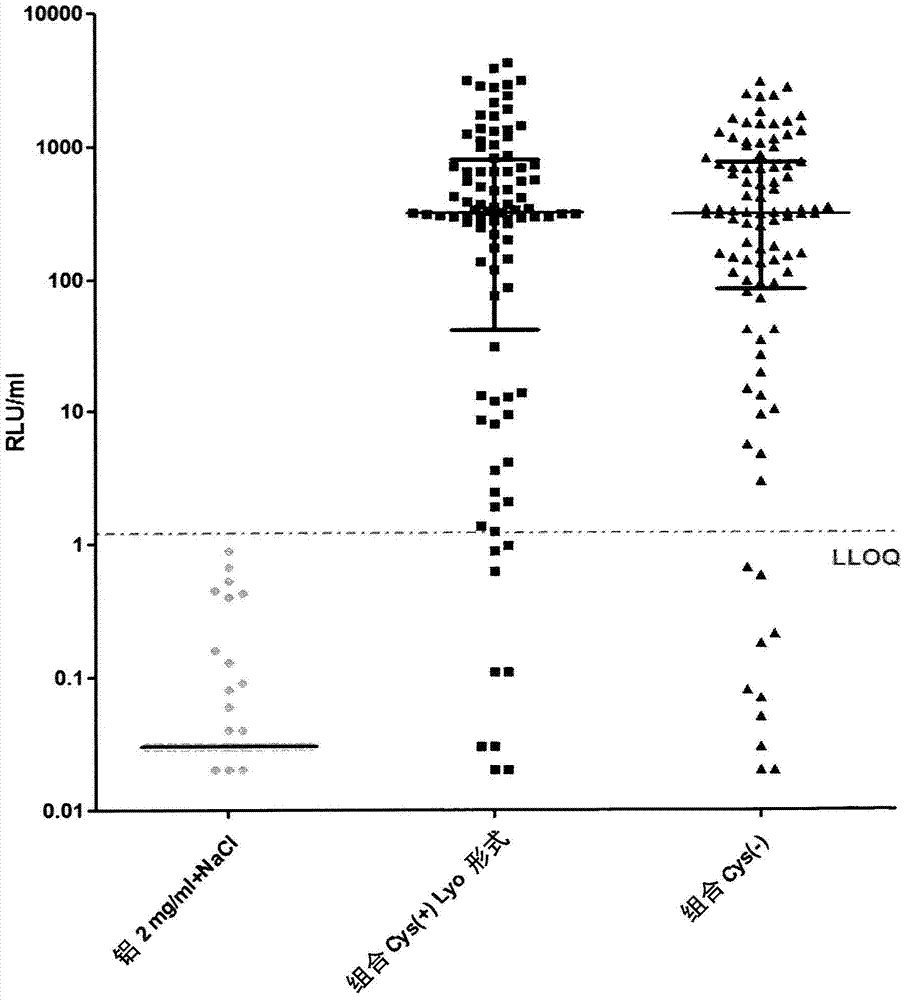
[0252] Immunogenicity studies in mice
[0253]The immunogenicity of cysteine-containing (Cys(+)) S. aureus antigens was compared to the corresponding cysteine-deficient (Cys(-)) variants. Five-week-old CD1 mice were immunized intraperitoneally with 14-day interval prime-boost injections of purified recombinant protein adsorbed to aluminum hydroxide adjuvant (Aluminum, 2 mg / ml). Mice were divided into 3 groups: (1) "combined Cys(+)Lyo format": with quadrivalent vaccine containing EsxAB-Cys(+), Sta006Cys(+), Sta011Cys(+), HlaH35L and aluminum hydroxide adjuvant Immunized mice; (2) "combined Cys(-)": mice immunized with quadrivalent vaccine containing EsxAB Cys(-), Sta006Cys(-), Sta011Cys(-), HlaH35L and aluminum hydroxide adjuvant; and (3) "Aluminum 2 mg / ml+NaCl": control mice receiving equal amounts of PBS and aluminum hydroxide adjuvant. Animals were bled immediately before the first immunization and 23 days later, and IgG antibodies against purified proteins were detected i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com