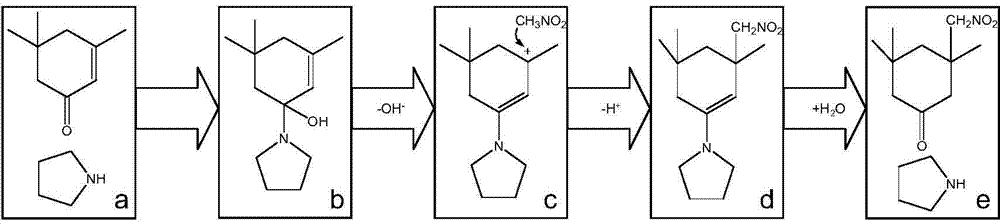
Method for preparing isophorone diamine
A technology of isophorone diamine and isophorone, applied in the field of preparing isophorone diamine, can solve the problems of low yield, long reflux time and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 Tetrabutylammonium fluoride as catalyst
[0037] In a 100ml glass bottle, add 10mmol of isophorone, 15mmol of nitromethane, and 1ml of tetrabutylammonium fluoride, react at 60°C for 1 hour, the conversion rate is 19%, and the conversion is 32% in 5 hours.
Embodiment 2
[0038] Embodiment 2 Tetrabutylammonium hydroxide as catalyst
[0039] In a 100ml glass bottle, add 10mmol of isophorone, 15mmol of nitromethane, and 5mmol of tetramethylammonium hydroxide, react at 70°C for 5 hours, and the conversion rate is 40%.
Embodiment 3
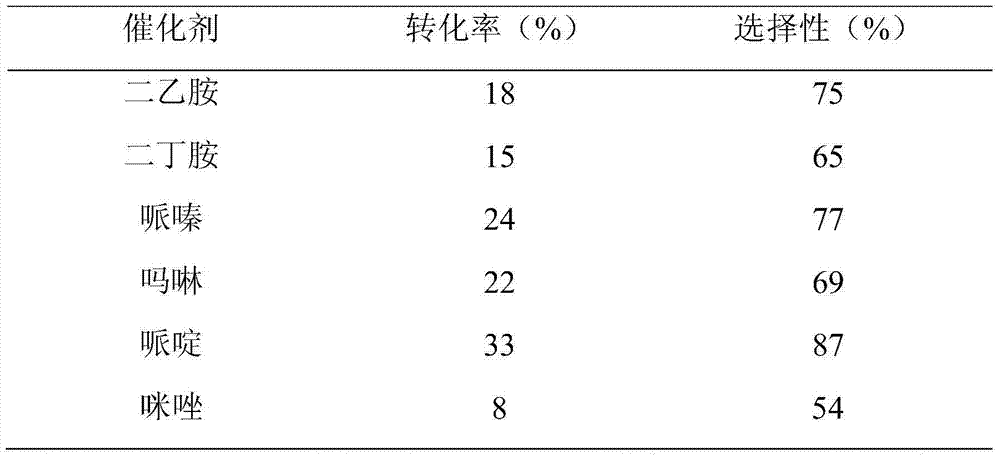
[0040] The catalytic effect of several secondary amines of embodiment 3
[0041]
[0042] Isophorone 10mmol, nitromethane 15mmol, catalyst 10mmol, reaction temperature 50°C.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap