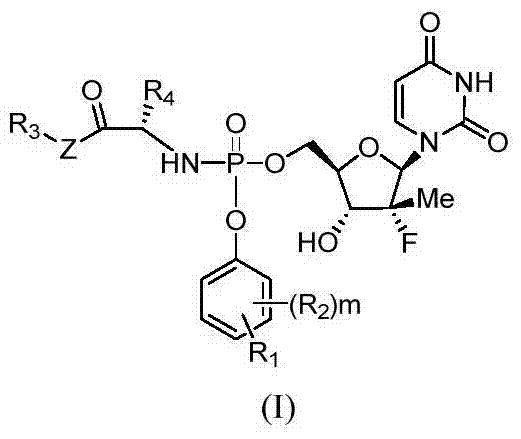
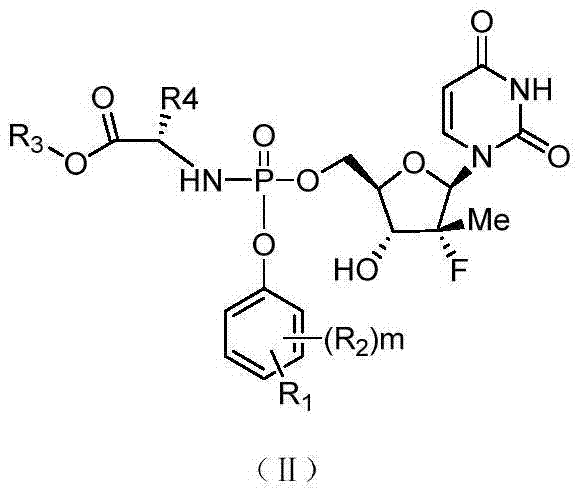
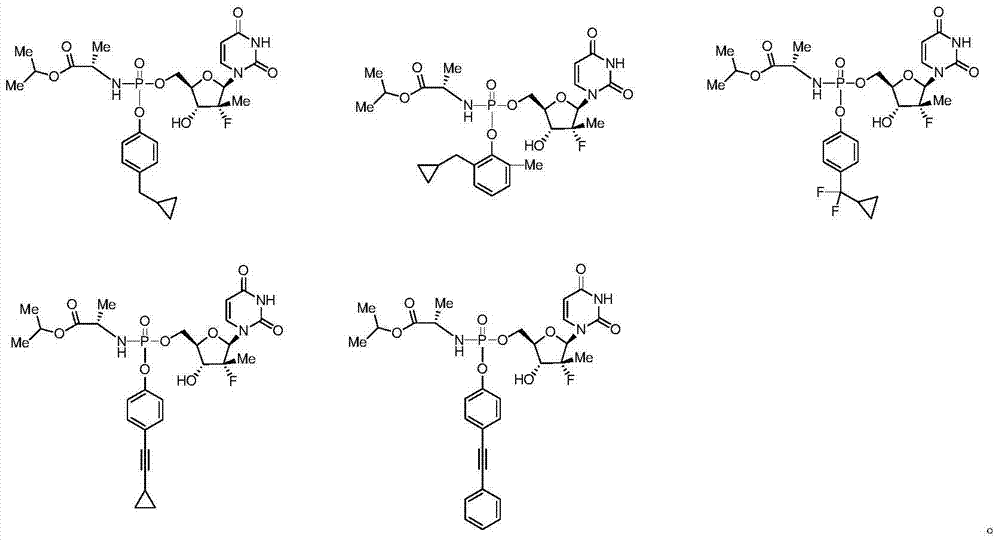
Uridine monophosphate analogue, and preparation method and applications thereof
A technology for uracil nucleotides and analogs, applied in the field of uracil nucleotide analogs and their preparation, can solve the problems of unfavorable virus clearance, anti-hepatitis C products on the market, too large molecules, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0143] The first step of preparation of isopropyl ((2-cyclopropylphenoxy)(4-nitrophenoxy)phosphoryl)-L-alanine ester
[0144]
[0145] 4-Nitrophenylphosphorus dichloride (320mg, 1.25mmol) was dissolved in CH 2 Cl 2 (2.5mL), cooled to -78°C, CH 2 Cl 2 (2.5mL) solution was added dropwise, reacted at this temperature for 30 minutes and gradually warmed up to 0°C, and the reaction solution was added dropwise to cooled L-alanine isopropyl ester hydrochloride (210mg , 1.25mmol) of CH 2 Cl 2 (2.5mL) solution, then TEA (366L, 2.63mmol) was added dropwise to the reaction system, stirred at 0°C for 1 hour, the reaction solution was concentrated under reduced pressure, EtOAc (20mL) was added to the reaction flask, the white solid was filtered, and the filtrate Concentration gave a yellow oily liquid. Column chromatography (eluent: PE:EtOAc=5:1) afforded the title compound isopropyl((2-cyclopropylphenoxy)(4-nitrophenoxy)phospho)-L-alanine acid ester (395mg, 70%).
[0146] 1 H ...
Embodiment 2
[0154] The first step of preparation of isopropyl ((3-cyclopropylphenoxy)(4-nitrophenoxy)phosphoryl)-L-alanine ester
[0155]
[0156] 4-Nitrophenylphosphorus dichloride (1.950g, 7.62mmol) was dissolved in CH 2 Cl 2 (15mL), cooled to -78°C, CH 2 Cl 2 (15mL) solution was added dropwise, reacted at this temperature for 30 minutes and gradually warmed up to 0°C, and the reaction solution was added dropwise to L-alanine isopropyl hydrochloride (1.279g ,7.63mmol) of CH 2 Cl 2 (15mL) solution, then TEA (2.23mL, 16.0mmol) was added dropwise to the reaction system, stirred at 0°C for 1 hour, the reaction solution was concentrated under reduced pressure, EtOAc (30mL) was added to the reaction flask, the white solid was filtered, and the filtrate Concentration gave a yellow oily liquid. Column chromatography (eluent: PE:EtOAc=4.5:1) afforded the title compound isopropyl((3-cyclopropylphenoxy)(4-nitrophenoxy)phospho)-L-alanine Ester (2.905g, 85%).
[0157] 1 H NMR (400MHz, CD...
Embodiment 3
[0165] The first step of preparation of isopropyl ((2-cyclopropyl-6-methylphenoxy)(4-nitrophenoxy)phosphoryl)-L-alanine ester
[0166]
[0167] 4-Nitrophenylphosphorous dichloride (1.950g, 7.62mmol) was dissolved in CH 2 Cl 2 (15mL), cooled to -78°C, CH 2 Cl 2 (15mL) solution was added dropwise, reacted at this temperature for 30 minutes and gradually warmed up to 0°C, and the reaction solution was added dropwise to L-alanine isopropyl hydrochloride (1.279g ,7.63mmol) of CH 2 Cl 2 (15mL) solution, then TEA (2.23mL, 16.0mmol) was added dropwise to the reaction system, stirred at 0°C for 1 hour, the reaction solution was concentrated under reduced pressure, EtOAc (30mL) was added to the reaction flask, the white solid was filtered, and the filtrate Concentration gave a yellow oily liquid. Column chromatography (eluent: PE:EtOAc=5:1~3:1) gave the title compound isopropyl ((2-cyclopropyl-6-methylphenoxy)(4-nitrophenoxy )phospho)-L-alaninate (2.880 g, 82%).
[0168] 1 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com