A kind of 2,2'-bis(benzothiophene)ethylene polymer and its preparation method and application
A technology of polymers and compounds, applied in the manufacture of semiconductor/solid-state devices, electrical components, circuits, etc., can solve the problem of less benzothiophene systems, achieve good air stability, and achieve simple and efficient synthesis routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
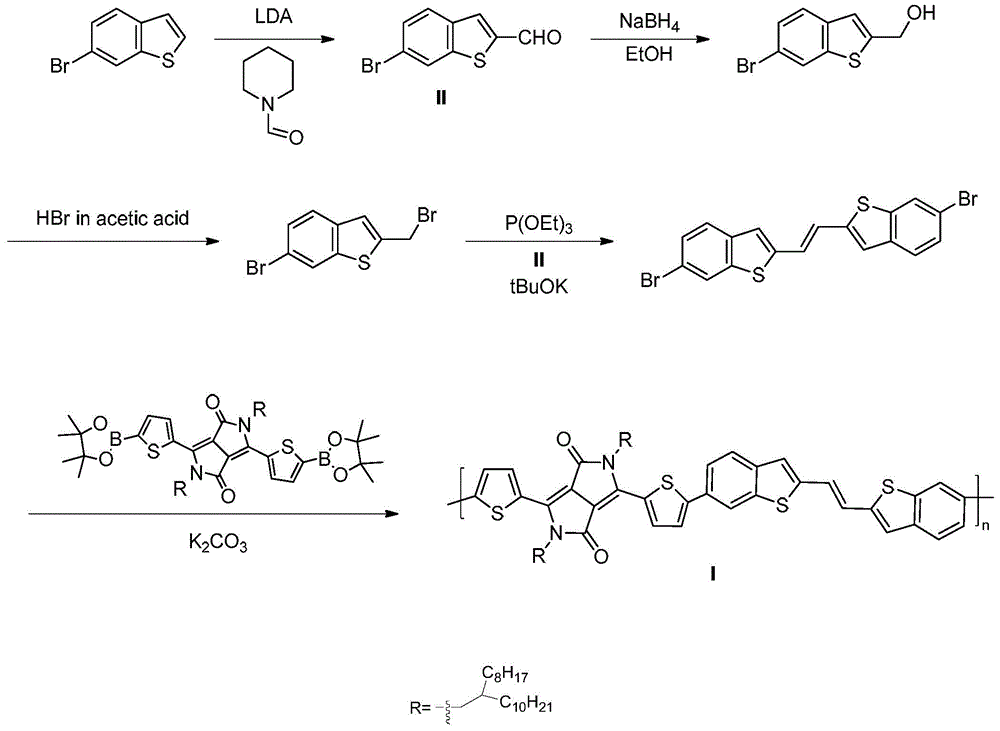
[0060] Example 1. Poly{2,5-di(2-octyldodecyl)-2,5-dihydro-1,4-dioxopyrrolo[3,4-c]pyrrole-3,6 -Diylthiophene-2,5-diyl-(E)-1,2-bis(benzo[b]thiophen-2-yl)ethylene-6,6'-diylthiophene-2,5-diyl }(P6BTE)(n=9)
[0061] The synthetic route of polymer P6BTE is as follows figure 1 As shown, the specific operation is as follows:
[0062] 1) Synthesis of 6-bromo[1]benzothiophene-2-carbaldehyde
[0063] Dissolve 6-bromo[1]benzothiophene (3.8g, 17.83mmol) in anhydrous THF (100mL), and slowly add fresh LDA (2.0M, 9.5mL, 19.0mmol) dropwise at 0°C, the reaction mixture After stirring at this temperature for 30 min, N-formylpiperidine (2.15 g, 19.0 mmol) was added dropwise. After warming to room temperature and reacting for 3 h, it was quenched with saturated ammonium chloride, extracted with dichloromethane, and dried over magnesium sulfate. The solution was spun off, and the mixture was subjected to silica gel column chromatography (eluent petroleum ether / ethyl acetate, v / v 10:1) to obtain...
Embodiment 2
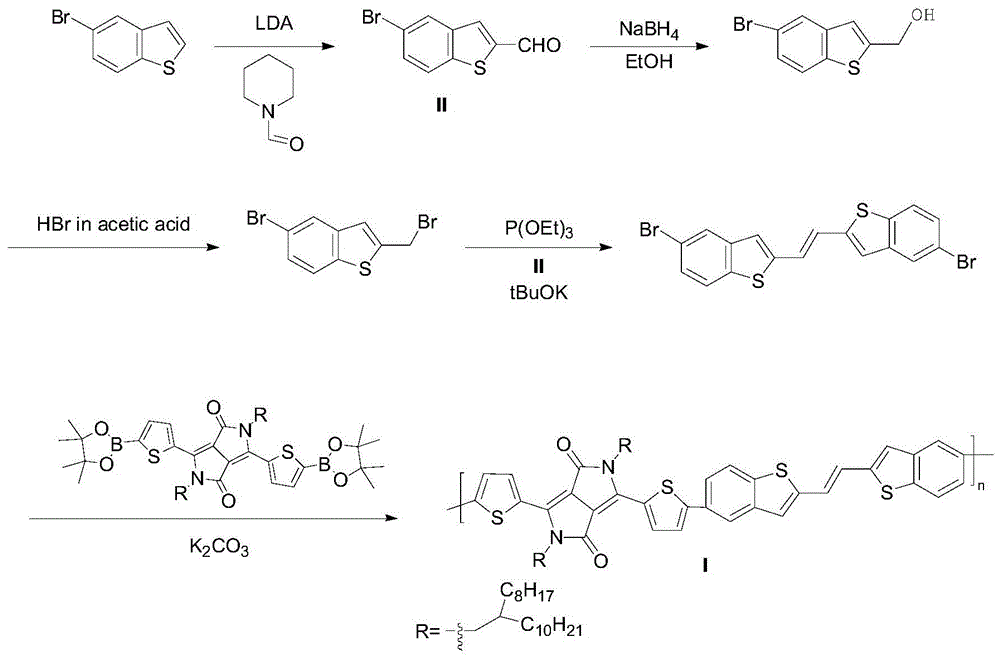
[0088] Example 2, poly{2,5-di(2-octyldodecyl)-2,5-dihydro-1,4-dioxopyrrolo[3,4-c]pyrrole-3,6 -Diylthiophene-2,5-diyl-(E)-1,2-bis(benzo[b]thiophen-2-yl)ethylene-5,5'-diylthiophene-2,5-diyl }(compound P5BTE)
[0089] 1) Synthesis of 5-bromo[1]benzothiophene-2-carbaldehyde
[0090] 5-Bromo[1]benzothiophene (3.8g, 17.83mmol) was dissolved in anhydrous THF (100mL), and fresh LDA (2.0M, 9.5mL, 19.0mmol) was slowly added dropwise at 0°C, and the reaction mixture After stirring at this temperature for 30 min, N-formylpiperidine (2.15 g, 19.0 mmol) was added dropwise. After warming to room temperature and reacting for 3 h, it was quenched with saturated ammonium chloride, extracted with dichloromethane, and dried over magnesium sulfate. The solution was spun off, and the mixture was subjected to silica gel column chromatography (eluent petroleum ether / ethyl acetate, v / v 10:1) to obtain 3.22 g of a light yellow solid. Yield: 75%.
[0091] The structural characterization data are as ...
Embodiment 3
[0115] Spectral performance and field effect transistor performance of embodiment 3, P6BTE and P5BTE
[0116] 1) Spectral properties of P6BTE and P5BTE
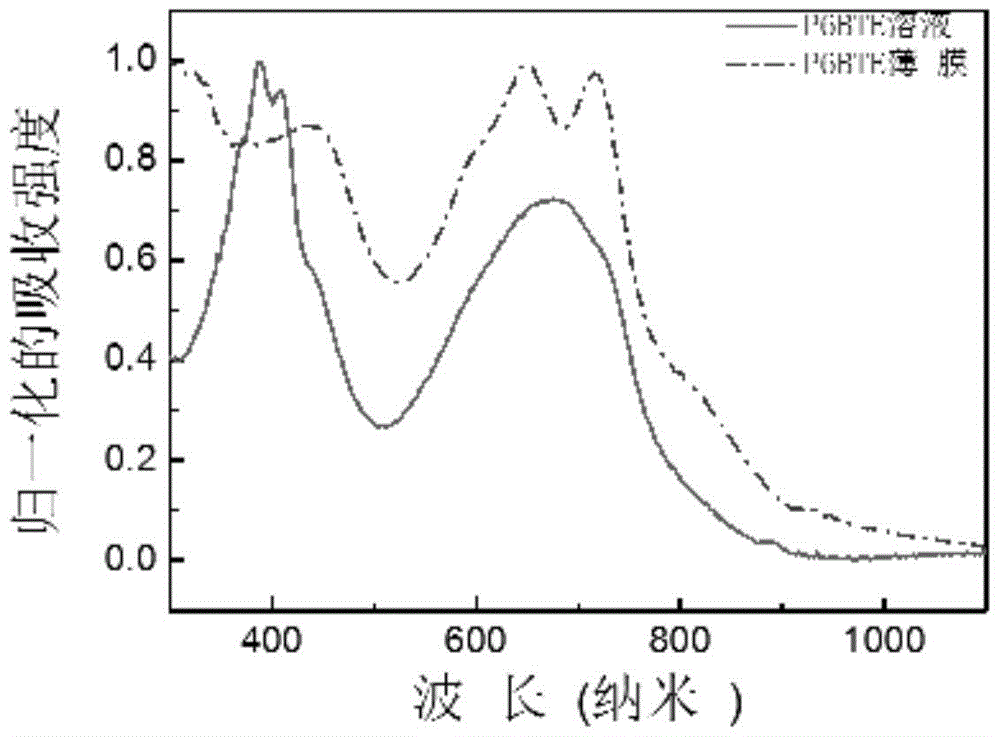
[0117] image 3 UV-Vis absorption spectra of P6BTE chloroform solution and film.
[0118] Figure 4 UV-Vis absorption spectra of P5BTE chloroform solution and film.
[0119] Depend on image 3 It can be seen that the absorption peak position of the polymer P6BTE in chloroform is about 674 nanometers, and the film absorption peak positions are at 650 and 716 nanometers, and the optical bandgap calculated according to its film absorption sideband is 1.37 electron volts (the optical bandgap is based on the formula E. g =1240 / λ calculation, where E g is the optical band gap, and λ is the boundary value of the UV absorption curve).
[0120] Depend on Figure 4 It can be seen that the absorption peak position of the polymer P5BTE in chloroform is about 684 nanometers, and the absorption peak position of the film is at 676 n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com