Application of polygonum cuspidatum in preparation for medicine for preventing and treating ionization radiation-induced intestinal injuries
A technology of small intestinal damage and ionizing radiation, applied in the preparation of anti-radiation-induced small intestinal damage inhibitory drugs, the application field of radiation-induced small intestinal damage drugs, to achieve the effect of a wide range of sources and significant small intestinal radiation damage protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Main reagents and experimental animals
[0029] Polygonum cuspidatum is purchased from the market and prepared by itself: crush the medicine, pass through an 80-mesh sieve, mix well, add 2ml of 95% alcohol to each gram of medicine powder, soak for 1 week, filter under sterile pressure, and soak in the same amount of alcohol After 1 week, pressurize and filter under sterile conditions, and evaporate the alcohol in a vacuum to evaporate the remaining powder for later use. During the experiment, 0.5% sodium carboxymethylcellulose solution was used to help dissolve and prepare a suspension. Cleaved Caspase-3 monoclonal antibody is a product of RD Company in the United States, the clone number is 269518; the Ki67 polyclonal antibody is a product of Abcam Company in the United States, the product number is ab66155. Hematoxylin, eosin, and immunohistochemical staining kits (secondary antibodies, DAB, etc.) are all products of Beijing Zhongshan Jinqiao. C57BL / 6 male mice ...
Embodiment 2
[0041] Experimental Results and Discussion
[0042] (1) Cytotoxicity to small intestinal mucosa
[0043] Mouse intestinal mucosal cells were cultured in vitro, and the cell concentration was adjusted to 2×10 6 / ml. Polygonum cuspidatum extract medium with different concentrations was prepared. 100 μl of cell suspension and different concentrations of treatment drugs were added to 96-well culture plates, and after incubation for 18 hours, cell viability was measured by bioluminescence. It was found that the extract of Polygonum cuspidatum at a concentration lower than 10 -4 No obvious cytotoxic effect was found at M. See Table 1 and Table 2 for the results.
[0044] Table 1 Effect of Polygonum cuspidatum on the viability of small intestinal mucosal cells
[0045]
[0046] Table 2 Effect of Polygonum cuspidatum on the viability of small intestinal mucosal cells
[0047]
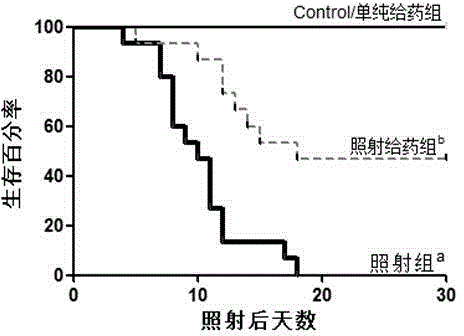
[0048] (2) Protective effect on mouse small intestinal mucosal cells from radiation injury in...
Embodiment 3
[0069] Preparation of traditional Chinese medicine Polygonum cuspidatum:
[0070] The preparation method is as follows: pulverize the traditional Chinese knotweed knotweed, pass through an 80-mesh sieve, mix evenly, add 2ml of 95% alcohol to each gram of medicine powder and soak for 1 week, filter under sterile pressure, and then soak in the same amount of alcohol for 1 week. Bacteria conditions and then pressurized filtration, after vacuum volatilization of alcohol, the remaining powder is ready for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com