Adipic acid bis-imidazoline derivative and preparation method thereof, and applications of adipic acid bis-imidazoline derivative as corrosion inhibitor
A technology of adipic acid and imidazoline, applied in the field of adipic acid bisimidazoline derivatives, can solve the problems of unsatisfactory corrosion performance and low corrosion inhibition efficiency, and achieve excellent anti-CO2 corrosion performance, excellent corrosion inhibition performance, and improved corrosion resistance. water soluble effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
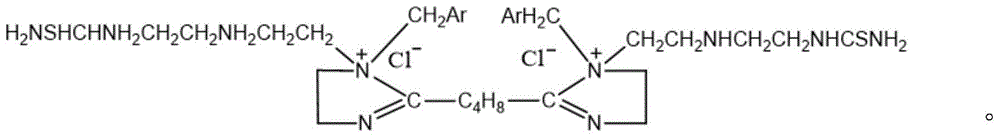
[0024] Compounds of the following structural formula:
[0025]
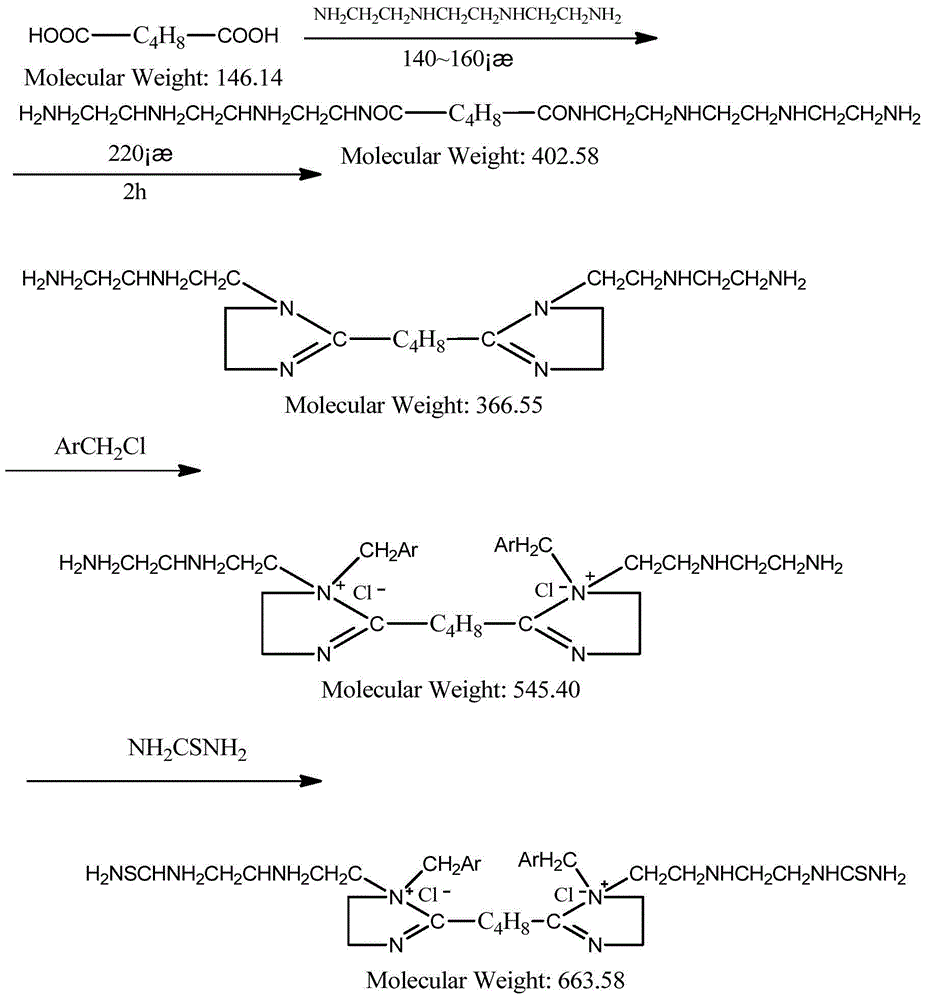
[0026] The preparation method of the above compound, the method comprises first reacting adipic acid and triethylenetetramine to generate an imidazoline intermediate, then reacting the imidazoline intermediate with benzyl chloride to obtain a quaternized product, and finally quaternizing The product reacts with thiourea to give the target compound.
[0027] Further, the method specifically includes the following steps:
[0028] (1) Dissolve 5.85g of adipic acid (0.04mol) in 20ml of xylene, the volume ratio of adipic acid to xylene is 1:1 (the volume is calculated by the relative density of adipic acid, and then added according to the volume ratio xylene);
[0029] (2) After the solution in step (1) is warmed up to 110°C, triethylenetetramine is added dropwise to the solution in step (1) according to the molar ratio of adipic acid and triethylenetetramine at 1:3, The specific amount of amine used is 17.55g. ...
preparation Embodiment 2
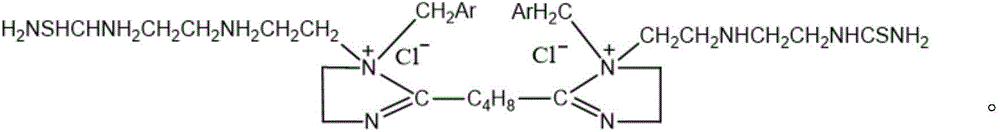
[0034] Compounds of the following structural formula:
[0035]
[0036] The preparation method of the above compound, the method comprises first reacting adipic acid and triethylenetetramine to generate an imidazoline intermediate, then reacting the imidazoline intermediate with benzyl chloride to obtain a quaternized product, and finally quaternizing The product reacts with thiourea to give the target compound.
[0037] Further, the method specifically includes the following steps:
[0038] (1) Dissolve 5.85g of adipic acid in 20ml of xylene, the volume ratio of adipic acid to xylene is 1:1 (calculate its volume based on the relative density of adipic acid, and then add xylene according to the volume ratio);
[0039] (2) After the solution in step (1) is warmed up to 115°C, triethylenetetramine, triethylenetetramine is added dropwise to the solution in step (1) according to the molar ratio of adipic acid to triethylenetetramine at 1:2.5. The specific amount of amine used...
preparation Embodiment 3
[0044] Compounds of the following structural formula:
[0045]
[0046] The preparation method of the above compound, the method comprises first reacting adipic acid and triethylenetetramine to generate an imidazoline intermediate, then reacting the imidazoline intermediate with benzyl chloride to obtain a quaternized product, and finally quaternizing The product reacts with thiourea to give the target compound.
[0047] Further, the method specifically includes the following steps:
[0048] (1) Dissolve 5.86g of adipic acid in 20ml of xylene, the volume ratio of adipic acid to xylene is 1:1 (calculate its volume based on the relative density of adipic acid, and then add xylene according to the volume ratio);
[0049] (2) After the solution in step (1) is warmed up to 105°C, triethylenetetramine, triethylenetetramine is added dropwise to the solution in step (1) according to the molar ratio of adipic acid to triethylenetetramine at 1:2.2. The specific amount of amine used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com