Recombinant human kallikrein
A technology of kalalidin and recombinant protein, which is applied in the field of recombinant human kalalidin, and can solve the problems that there are no high-molecular-weight recombinant human kalalidin research reports.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
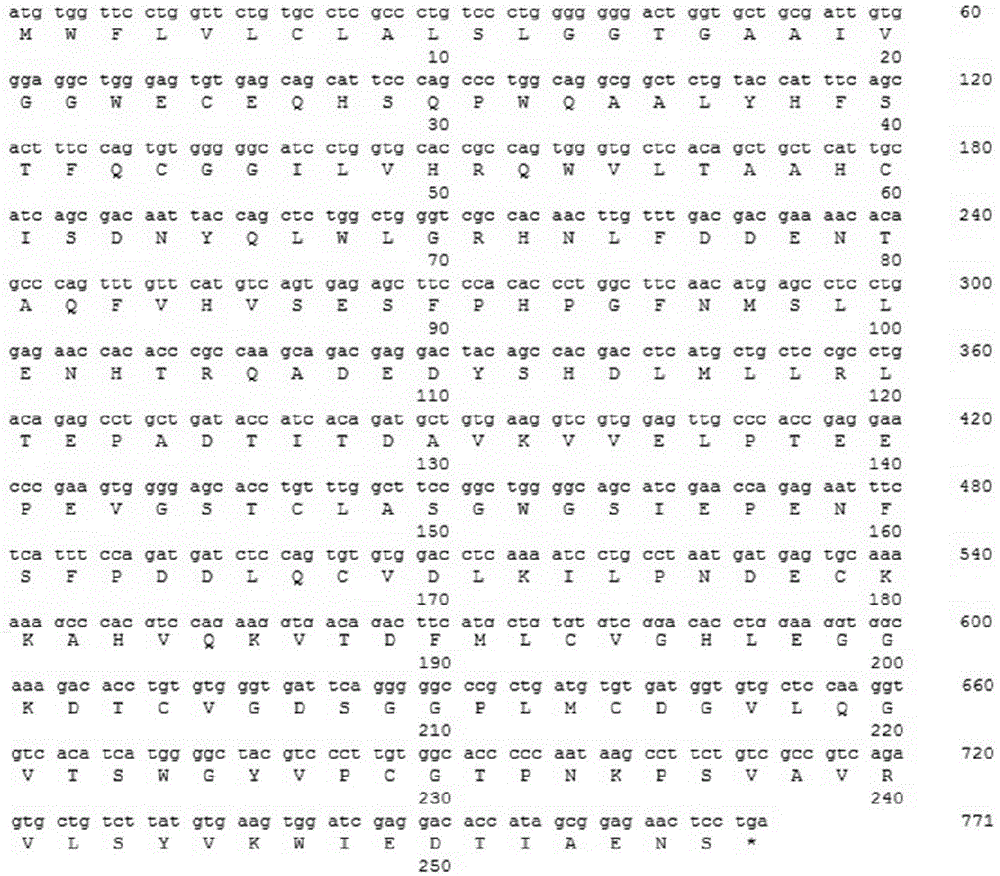
[0072] Example 1 Constructing the Gene Expression Vector Encoding the Human Kawailin Recombinant Protein
[0073] The leader peptide and mature protein gene encoding human kalalidin were obtained by artificial synthesis, with a full length of 771bp (sequence 1 and figure 1 ), this DNA fragment was inserted between the restriction enzyme site KpnI in the vector such as pUC57, and the plasmid pUC-human angioretin was obtained. The accuracy of the synthesized fragments was verified by DNA sequencing.
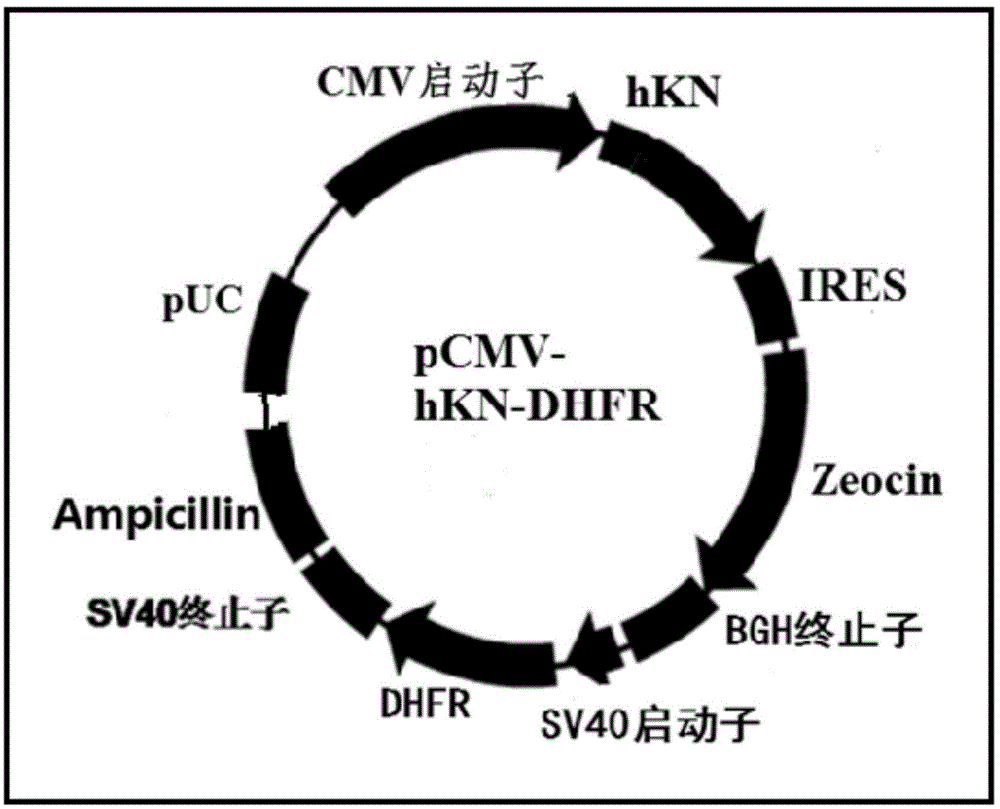
[0074] The mouse DHFR gene, promoter and terminator fragments containing restriction sites EcoRI (5' end) and HindIII (3' end) were obtained by artificial synthesis, and inserted into the mammalian cell expression vector pCMV / ZEO (Invitrogen ) in the corresponding restriction site, the vector pCMV-DHFR was obtained, and the sequences of DHFR, promoter and terminator contained in it could be verified by DNA sequencing.
[0075] The obtained complete gene sequence encoding human...
Embodiment 2
[0076] Example 2. Stable Expression of Recombinant Human Kalatin in Mammalian Host Cells
[0077] In order to express the recombinant human kalalidin protein, the constructed gene expression vector pBudCE4.1-DHFR-human kalalidin recombinant protein was transfected into a mammalian host cell line. The preferred host cell line is the DHFR enzyme-deficient CHO-cell (DHFR--CHO), and for the host cell to be more suitable for expressing the secreted protein, and the cell can reach a higher density, we will improve the DHFR--CHO cell, improve Methods include transfecting proteases and altering cellular metabolic pathways. The cell transfection method is preferably electroporation, but other methods including calcium phosphate co-sedimentation, lipofection, and protoplast fusion can also be used. In electroporation, add 10 μg of plasmid linearized with PvuI to 2–5×107 cells in a cuvette using a Gene Pulser Electroporator (Bio-Rad Laboratories, Hercules, CA) set at 250 V electric fiel...
Embodiment 3
[0079] Example 3. Cell production of recombinant human kalalin protein
[0080] The cell lines obtained in Example 2 were first adapted to suspension culture in shake flasks. Human kalalin is a glycosylated protein, and the glycosylation level of the protein is directly related to its activity and side effects after being made into a drug. For protein, we optimized the culture medium, and added some substances to increase the glycosylation level of recombinant human kalalidin through a comparative experiment in small shake flask culture. Add 100 μM Cu to basal medium T300 2+ , adding 2mM ManNAc (N-acetyl-D-aminomannose) to the feeding medium can increase the level of glycosylation. When the shake flask culture of the cells was stable, in order to obtain more human kalalin recombinant protein, the cells were expanded to a sufficient amount, and batch culture was carried out in a 7L bioreactor. The set parameters of the bioreactor are: temperature 37° C., pH 6.90, dissolved o...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap