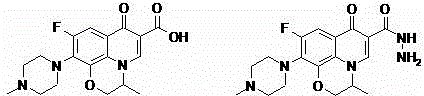
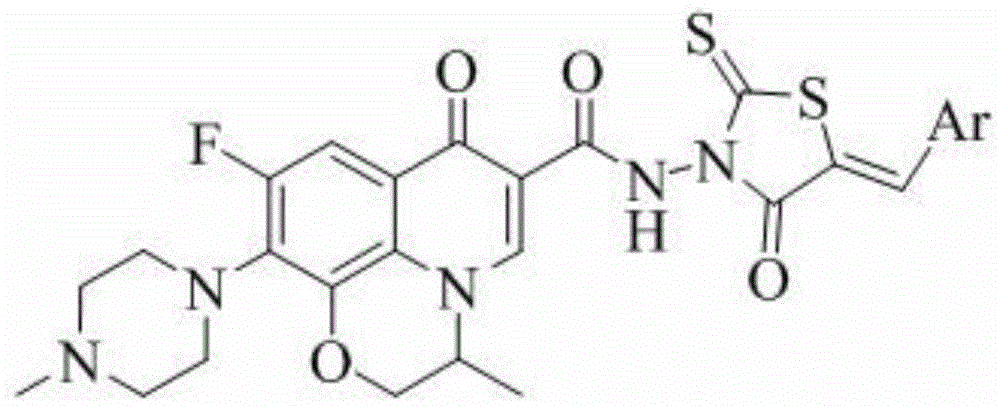
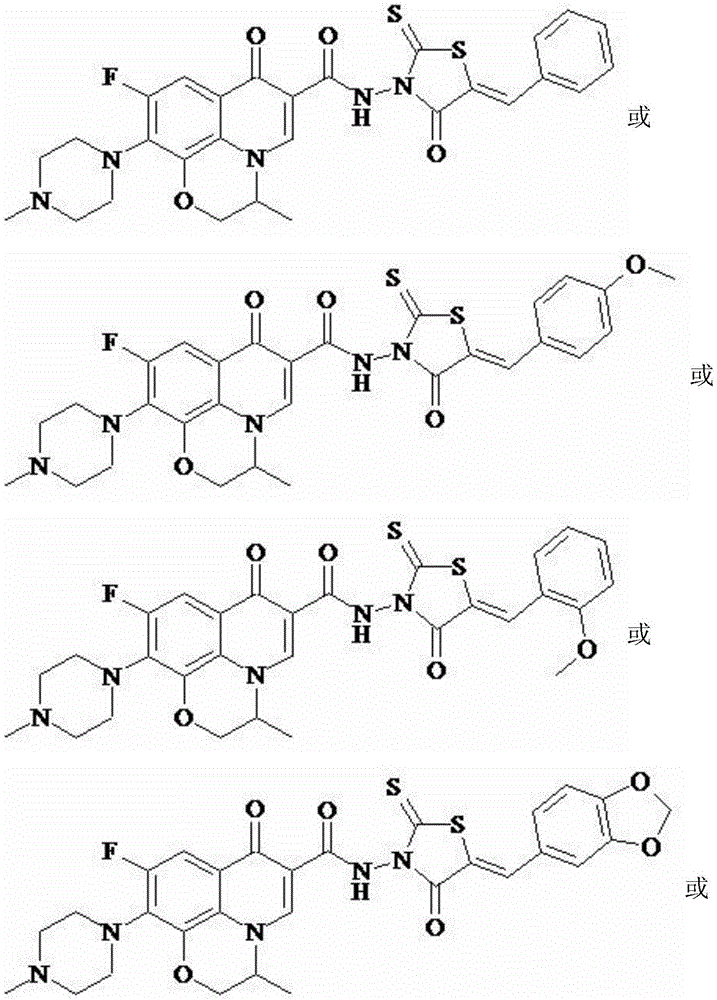
A kind of ofloxacin (rhodanine unsaturated ketone) amide derivative and its preparation method and application
A technology of amide derivatives, ofloxacin, used in drug combination, organic chemistry, antitumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Ofloxacin (5-benzylidene-rhodanine-3-yl)-amide (I-1), its chemical structural formula is:
[0046]
[0047] That is, Ar in formula I is phenyl.
[0048] The preparation method of this compound is: get ofloxacin (rhodanine-3-base)-amide (IV) 1.0g (2.0mmol) and anhydrous sodium acetate 0.20g (2.4mmol) are dissolved in 15mL glacial acetic acid, 0.25 g (2.4 mmol) of benzaldehyde was added, and the mixed reactant was refluxed for 12 h. The solvent was distilled off under reduced pressure, the residue was dissolved in water (20 mL), and 0.1 g of activated carbon was added to decolorize at 60° C. for 0.5 h, and filtered. The filtrate was basified with concentrated ammonia water to pH 9.0, extracted with chloroform (3×15 mL), and the combined organic phases were dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure to dryness, and recrystallized from ethanol-DMF (V:V=5:1) to obtain a light yellow crystal (I-1), with a yield of 72.8%, m.p.193...
Embodiment 2
[0050] Ofloxacin (5-p-methoxybenzyl-rhodanine-3-yl)-amide (I-2), its chemical structural formula is:
[0051]
[0052] That is, Ar in formula I is p-methoxyphenyl.
[0053] The preparation method of this compound is: get ofloxacin (rhodanine-3-base)-amide (IV) 1.0g (2.0mmol) and anhydrous sodium acetate 0.20g (2.4mmol) are dissolved in 15mL glacial acetic acid, 0.30 g (2.2 mmol) of p-methoxybenzaldehyde was added, and the mixed reactant was refluxed for 12 h. The solvent was distilled off under reduced pressure, the residue was dissolved in water (20 mL), and 0.1 g of activated carbon was added to decolorize at 60° C. for 0.5 h, and filtered. The filtrate was basified with concentrated ammonia water to pH 9.0, extracted with chloroform (3×15 mL), and the combined organic phases were dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure to dryness, and recrystallized from ethanol-DMF (V:V=5:1) to obtain a light yellow crystal (I-2), with a y...
Embodiment 3
[0055] Ofloxacin (5-o-methoxybenzyl-rhodanine-3-yl) amide (I-3), its chemical structural formula is:
[0056]
[0057] That is, Ar in formula I is o-methoxyphenyl.
[0058] The preparation method of this compound is: get ofloxacin (rhodanine-3-base) amide (IV) 1.0g (2.0mmol) and anhydrous sodium acetate 0.20g (2.4mmol) and dissolve in 15mL glacial acetic acid, add 0.30 g (2.2 mmol) of o-methoxybenzaldehyde, and the mixed reactants were refluxed for 12 hours. The solvent was distilled off under reduced pressure, the residue was dissolved in water (20 mL), and 0.1 g of activated carbon was added to decolorize at 60° C. for 0.5 h, and filtered. The filtrate was basified with concentrated ammonia water to pH 9.0, extracted with chloroform (3×15 mL), and the combined organic phases were dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure to dryness, and recrystallized from ethanol-DMF (V:V=5:1) to obtain a light yellow crystal (I-3), with a yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com