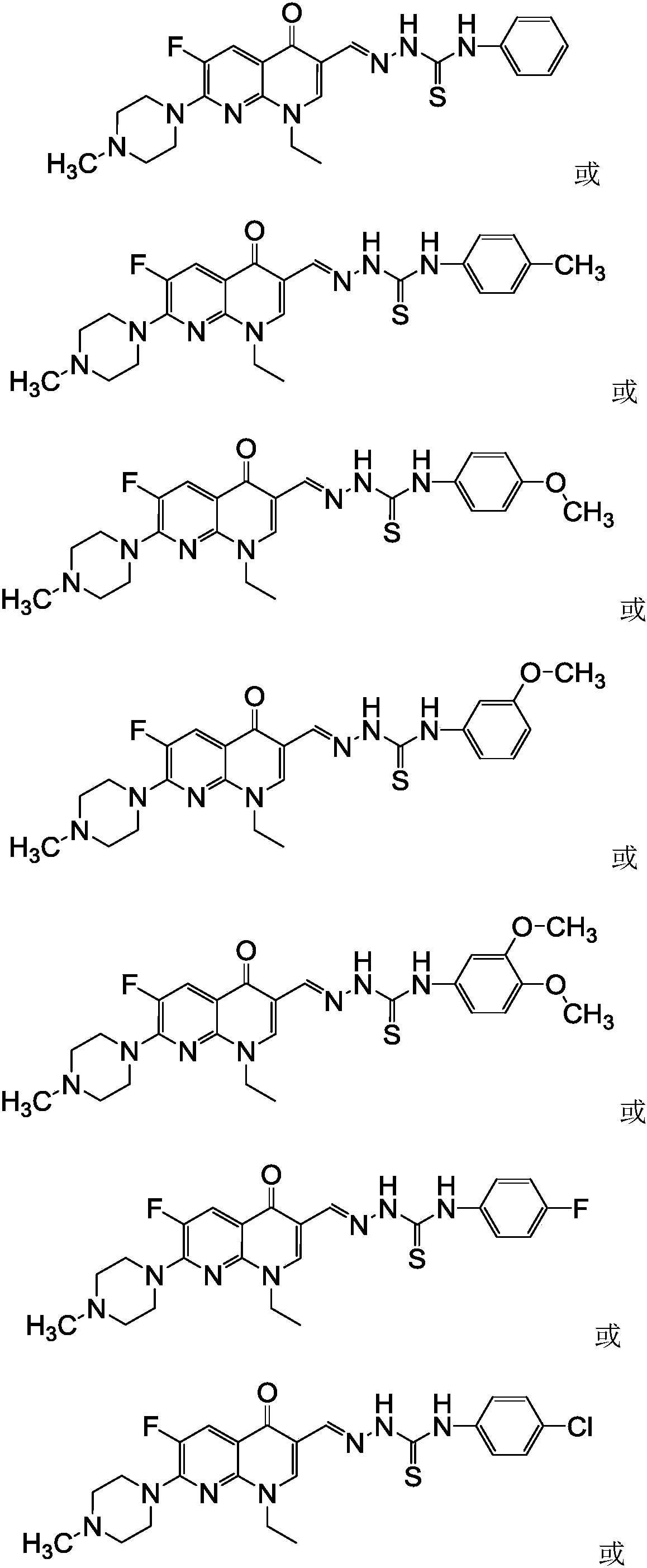
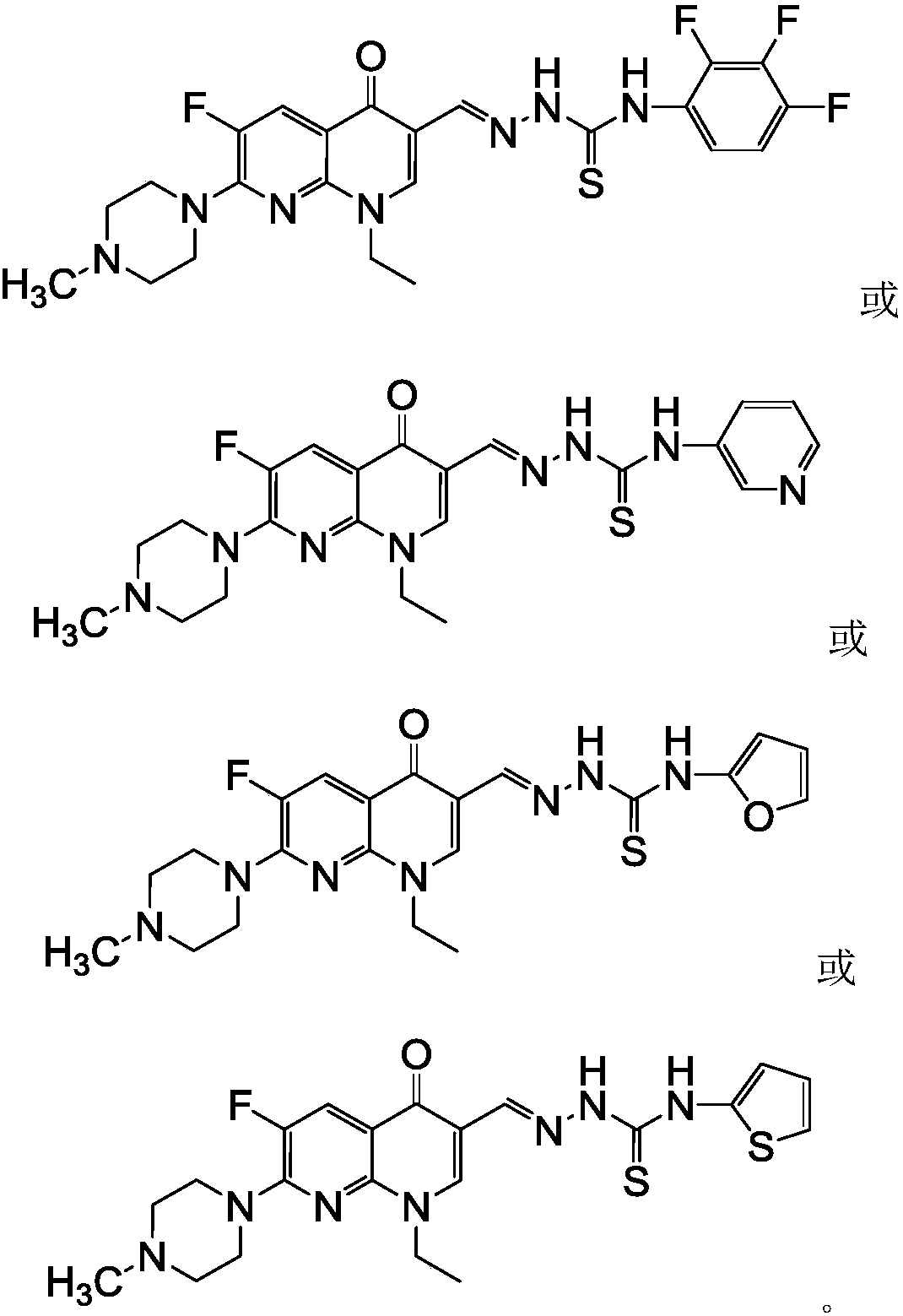
N-methyl enoxacin aldehyde acetal 4-aryl thiosemicarbazide derivatives and its preparation method and application
A technology of methylenoxacin aldehyde and methylenoxacin hydrazide, which is applied in the field of innovative drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
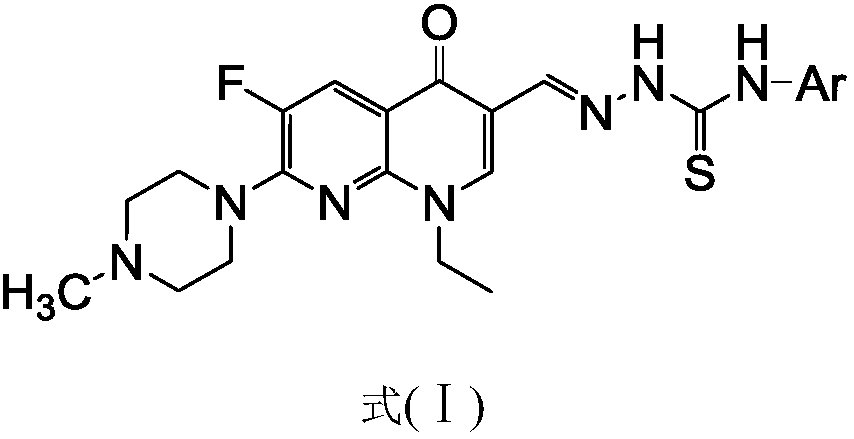
[0057] 1-Ethyl-6-fluoro-7-(4-methyl-piperazin-1-yl)-naphthyridin-4(1H)-one-3-aldehyde acetal 4-phenylthiosemicarbazide (I-1 ), its chemical structural formula (I-1) is:
[0058]
[0059] That is, the substituent Ar in formula I is phenyl.
[0060] The preparation method of this compound is: the N-methyl enoxacin C-3 aldehyde crude product (1.0g) shown in formula (V) is dissolved in dehydrated alcohol (20 milliliters), adds 4-phenylthiosemicarbazide ( 0.6g, 3.6mmol) (that is, Ar in the 4-aryl thiosemicarbazides shown in formula (IX) is phenyl), refluxed for 10 hours, filtered while hot and collected the solid, and the gained solid was washed successively with ethanol for 2 Wash twice with distilled water, dry, and recrystallize with a mixed solvent of DMF-ethanol with a volume ratio of 5:3 to obtain a yellow crystal with a structural formula (I-1) and a mass of 0.76g, m.p.238-240°C ;
[0061] 1 H NMR (400MHz, DMSO-d 6 )δ: 11.78(s, 1H, CH=N), 10.06(s, 1H, NH), 9.16(s, 1H...
Embodiment 2
[0064] 1-Ethyl-6-fluoro-7-(4-methyl-piperazin-1-yl)-naphthyridin-4(1H)-one-3-aldehyde acetal 4-(4-methylphenyl)amino Thiourea (I-2), its chemical structural formula (I-2) is:
[0065]
[0066] That is, Ar in formula I is 4-methylphenyl.
[0067] The preparation method of this compound is: N-methyl enoxacin C-3 aldehyde crude product (1.0g) shown in formula (V) is dissolved in dehydrated alcohol (20 milliliters), adds 4-(4-methylbenzene Base) thiosemicarbazide (0.6g, 3.3mmol) (that is, Ar in the 4-aryl thiosemicarbazides shown in formula (IX) is 4-methylphenyl), reflux reaction for 12 hours, filtered while hot and Collect the solid, wash the obtained solid twice with ethanol and distilled water twice, dry, and recrystallize with a mixed solvent of DMF-ethanol with a volume ratio of 5:3 to obtain a light yellow crystal, the structural formula of which is shown in formula (I-2) , mass is 0.68g, m.p.218~220℃;
[0068] 1 H NMR (400MHz, DMSO-d 6 )δ: 11.77(s, 1H, CH=N), 9.82(...
Embodiment 3
[0071] 1-Ethyl-6-fluoro-7-(4-methyl-piperazin-1-yl)-naphthyridin-4(1H)-one-3-aldehyde acetal 4-(4-methoxyphenyl) Thiosemicarbazide (I-3), its chemical structural formula (I-3) is:
[0072]
[0073] That is, Ar in formula I is 4-methoxyphenyl.
[0074] The preparation method of this compound is: N-methyl enoxacin C-3 aldehyde crude product (1.0g) shown in formula (V) is dissolved in dehydrated alcohol (20 milliliters), adds 4-(4-methoxy Phenyl) thiosemicarbazide (0.7g, 3.6mmol) (that is, Ar in the 4-aryl thiosemicarbazides shown in formula (IX) is 4-methoxyphenyl), reflux reaction for 8 hours, while hot Filtrate and collect the solid, the gained solid is washed with ethanol twice, distilled water twice successively, dry, use the mixed solvent recrystallization of the DMF-ethanol that is 5:3 with the volume ratio, obtain pale yellow crystal, structural formula is as formula (I- 3), the mass is 0.64g, m.p.234~236℃;
[0075] 1 H NMR (400MHz, DMSO-d 6 )δ: 11.78(s, 1H, CH=N)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com