A kind of two-dimensional conjugated benzodithiophene compound and its preparation method and use
A benzodithiophene, two-dimensional conjugation technology, used in organic chemistry, semiconductor/solid-state device manufacturing, photovoltaic power generation, etc., can solve problems such as inability to meet practical applications, achieve excellent photovoltaic performance, improve photovoltaic performance, and be easier to purify Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1. Synthesis of two-dimensional conjugated benzodithiophene compound SMBDTT-S.
[0042] 1. Synthesis of intermediate M1:
[0043] At room temperature, add A donor 2-[5-(5-bromo-3-n-octylthiophen-2-yl)thiophen-2-yl]-3-n-octylthiophene-5 -Formaldehyde (1.16g, 2mmol), B donor 1,6-bis(trimethyltinyl)-4,8-bis[5-(2-ethylhexylthio)thiophen-2-yl]benzo [1,2-b:4,5-b']dithiophene (0.97g, 1mmol), catalyst tetrakis(triphenylphosphine) palladium (47mg, 0.04mmol) and solvent toluene (50mL), after mixing and stirring , Refluxed at 110° C. for 24 hours under nitrogen protection. After the reaction was completed, the reaction system was cooled to room temperature, the solvent was evaporated in vacuo, and purified by a silica gel column using dichloromethane as the eluent to obtain a reddish-brown solid M1 (1 g, yield: 63%).
[0044] 1 H-NMR (400 MHz, CDCl 3): δ (ppm) 9.83 (s, 2H), 7.60-7.59 (d, 4H),7.35-7.34 (d, 2H), 7.26-7.25 (d, 4H), 7.13 (s, 4H), 3.00-2.99 (d, 4H), 2.85-...
Embodiment 2
[0050] Example 2: Research on photovoltaic properties of SMBDTT-S.
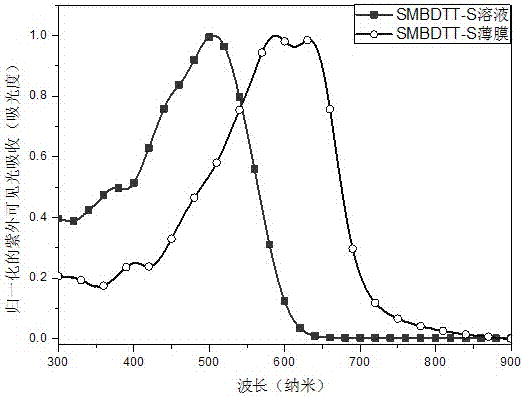
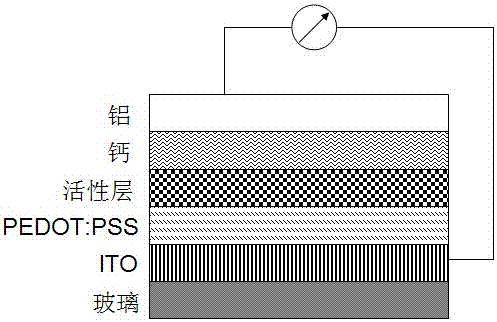
[0051] With SMBDTT-S as the donor, PC 70 BM is the acceptor, and the organic solar cell device is prepared by solution spin coating, and its structure schematic diagram is shown in figure 2 As shown, the layered structure of the device from bottom to top is ITO / PEDOT:PSS / active layer (SMBDTT-S:PC 70 BM) / Ca / Al.
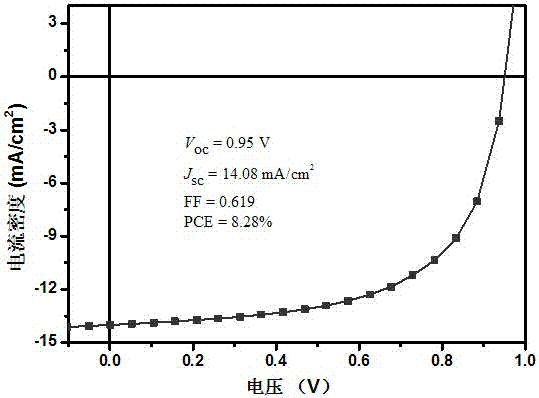
[0052] Based on SMBDTT-S / PC 70 BM (weight ratio 1:0.8) organic solar cell device is the research object, and its photovoltaic properties are investigated. The current-voltage curve is as follows image 3 shown. It can be seen that the open circuit voltage of the device ( V oc ) is 0.95V, the short-circuit current ( J sc ) is 14.08mA / cm 2 , the fill factor (FF) is 0.619, and the power conversion efficiency (PCE) is 8.28%. Unlike the high-efficiency small-molecule materials reported in the literature, the photovoltaic performance is achieved without the use of any additives, solvent annealing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com