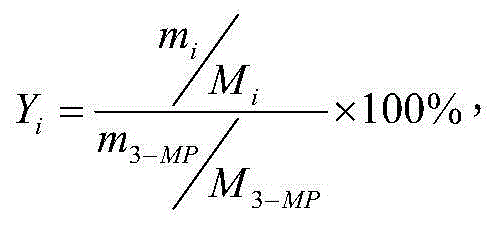Catalyst for preparing 3-trifluoromethyl pyridine
A technology of trifluoromethylpyridine and methylpyridine, which is applied in the field of preparation of 3-trifluoromethylpyridine, can solve the problems of poor selectivity of 3-trifluoromethylpyridine, large reaction energy consumption, etc., and achieves improved activity and Selectivity, low reaction temperature, the effect of improving selectivity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Catalyst preparation
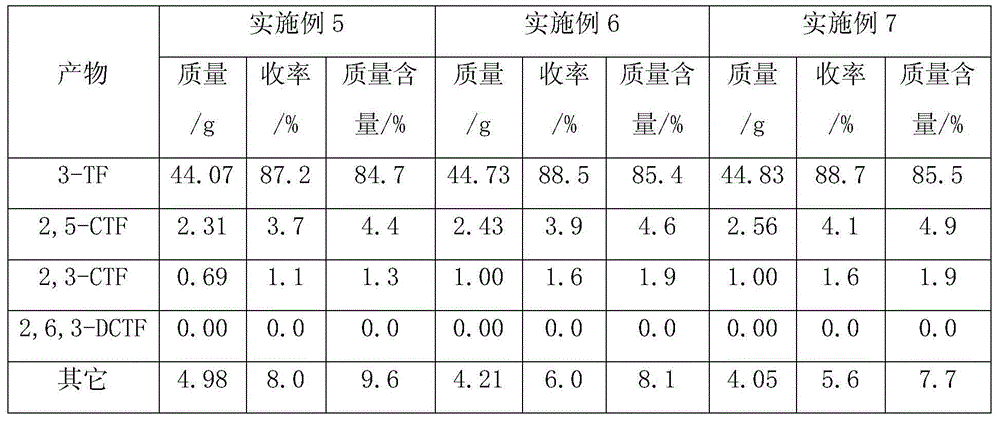
[0032] The inner diameter of the reaction tube is 19mm, the length is 700mm, the material is stainless steel, and the catalyst filling height is 140mm. The catalyst bed is composed of 55.5%MgF 2 -40.0%Co 2 O 3 -0.55% CeO 2 (55.5%, 40%, 0.5% are the mole percentages of metal atoms, which are the ratio of the number of moles of metal atoms of each component to the total number of moles of metal atoms, the same below), the catalyst is formed into a diameter of 3mm and a height of 4mm Of the cylinder. Heat the reaction tube to 235°C, control the feed rate of anhydrous hydrogen fluoride to 10.00g / h (0.500mol / h), pass in HF for 3 hours to activate the catalyst, and then use nitrogen as the carrier gas to vaporize 3-methyl Pyridine and chlorine gas are introduced into the reaction tube. Among them, the flow rate of 3-picoline is controlled to 4.00g / h (0.043mol / h), the flow rate of chlorine is controlled to 6.0L / h (0.268mol / h), and the flow rate ...
Embodiment 2
[0037] Fill the reaction tube described in Example 1 with 55.5% MgF 2 -40%ZnO-0.5%K 2 O catalyst, the catalyst is shaped into a cylinder with a diameter of 3mm and a height of 4mm. Heat the reaction tube to 260°C, control the feed rate of anhydrous hydrogen fluoride to 10.00g / h (0.500mol / h), pass in HF for 3 hours to activate the catalyst, and then use nitrogen as the carrier gas to vaporize 3-methyl The pyridine and chlorine gas are introduced into the reaction tube. Among them, the flow rate of 3-picoline is controlled to 4.00g / h (0.043mol / h), the flow rate of chlorine is controlled to 6.0L / h (0.268mol / h), and the flow rate of nitrogen is maintained at 12.0L / h (0.536mol / h). / h). The molar feed ratio of the reactants is 3-picoline:chlorine gas:hydrogen fluoride:nitrogen=1:6.2:11.6:12.5, and the reaction is 8 hours.
[0038] The exhaust gas leaving the reaction tube was treated as in Example 1. 51.80 g of oily product was obtained, which was analyzed by gas chromatography. The...
Embodiment 3
[0043] Fill the reaction tube described in Example 1 with 77.0% MgF 2 -20.0%Bi 2 O 3 -2.0%Na 2 O catalyst, the catalyst is shaped into a cylinder with a diameter of 3mm and a height of 4mm. Heat the reaction tube to 220°C, control the feed rate of anhydrous hydrogen fluoride to 10.00g / h (0.500mol / h), pass in HF for 3 hours to activate the catalyst, and then use nitrogen as the carrier gas to vaporize 3-methyl The pyridine and chlorine gas are introduced into the reaction tube. Among them, the flow rate of 3-picoline is controlled to 4.00g / h (0.043mol / h), the flow rate of chlorine is controlled to 6.0L / h (0.268mol / h), and the flow rate of nitrogen is maintained at 12.0L / h (0.536mol / h). / h). The molar feed ratio of the reactants is 3-picoline:chlorine gas:hydrogen fluoride:nitrogen=1:6.2:11.6:12.5, and the reaction is 8 hours.
[0044] The exhaust gas leaving the reaction tube was treated as in Example 1. 51.91 g of oily product was obtained, which was analyzed by gas chromatogr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap