Preparation method and application of nitrofuran molecularly imprinted polymer microspheres
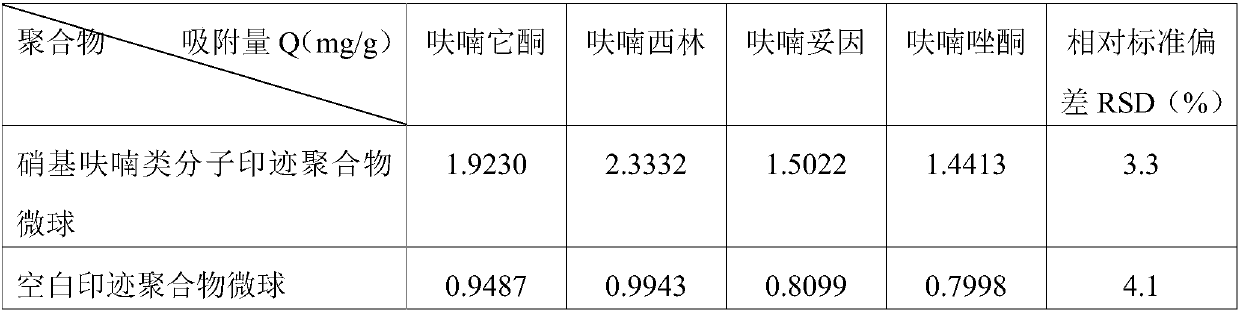
A technology of molecular imprinting and nitrofuran, applied in the field of medicine, can solve the problems of only one substance adsorbed, irregular particles of molecularly imprinted polymer, etc., and achieve the effect of uniform size, regular particle shape and improved adsorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The preparation method of nitrofuran molecularly imprinted polymer microspheres adopts a sacrificial silica gel method to synthesize nitrofuran molecularly imprinted polymer microspheres. Specifically follow the steps below;
[0022] Step 1. According to the molar ratio of α-methacrylic acid:furaltadone and nitrofurazone molecules=6:1~8:1, dissolve in the solvent, ultrasonically assist the dissolution, then add a crosslinking agent, fill with argon, seal, Prepolymerize in the refrigerator for 16h to 22h, then transfer the prepolymer to a PP plastic column polymerization reactor, add column chromatography silica gel, the ratio of the mass of column chromatography silica gel to the template molecule is 1.2g:1mmol~ 2g: 1mmol and initiator, shake fully, fill with argon again to exhaust oxygen, and then seal;
[0023] Step 2. React the sealed mixture in a constant temperature water bath shaker at 60°C to 70°C for 20h to 24h, cool it down, put the mixture in the refrigerator...
Embodiment 1
[0031] Dissolve а-methacrylic acid, furaltadone and nitrofurazone in acetonitrile solvent at a molar ratio of 7:1, the volume ratio of furaltadone and nitrofurazone to acetonitrile is 1mmol:80mL, and then the power is 65W Ultrasound for 5min under the condition of ethylene glycol dimethacrylate, and the molar ratio of ethylene glycol dimethacrylate:furaltadone and nitrofurazone is 45:1; fill with argon gas to remove oxygen, and then seal, Prepolymerize in the refrigerator for 20 hours, then transfer the prepolymer to a PP plastic column polymerization reactor, then add azobisisobutyronitrile and column chromatography silica gel, the mass of column chromatography silica gel and the ratio of template molecules Be 1.5g: 1mmol, the amount of the substance of α-methacrylic acid and ethylene glycol dimethacrylate: the mass ratio of azobisisobutyronitrile is 135mmol: 1g; Charge into argon, seal; After sealing The mixture was reacted in a constant temperature water bath shaker at 65°C...
Embodiment 2
[0044] According to the molar ratio α-methacrylic acid: the ratio of furaltadone and nitrofurazone molecule=6:1 is dissolved in acetonitrile, the volume ratio of the amount of furaltadone and nitrofurazone to acetonitrile is 1mmol:70mL; Add ethylene glycol dimethacrylate again, and the molar ratio of ethylene glycol dimethacrylate: furaltadone and nitrofurazone is 40:1; fill with argon, seal, pre-polymerize in refrigerator for 16h, and then pre-polymerize The polymer is transferred to a PP plastic columnar polymerization reactor, and column chromatography silica gel and azobisisobutyronitrile are added. The quality of the column chromatography silica gel and the ratio of template molecules are 1.2g:1mmol, а-methacrylic acid The amount of substance and ethylene glycol dimethacrylate: the mass ratio of azobisisobutyronitrile is 130mmol: 1g; shake fully, fill with argon again to exhaust oxygen, and then seal; seal the sealed mixture at 60°C React in a constant temperature water b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com