The miRNA combination and its kit for diagnosing early liver cancer
A technology of early liver cancer and kits, applied in the field of medical biological detection, can solve problems such as unsuitable for clinical screening, unsatisfactory sensitivity and specificity, and difficulty in distinguishing benign lesions from tumors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
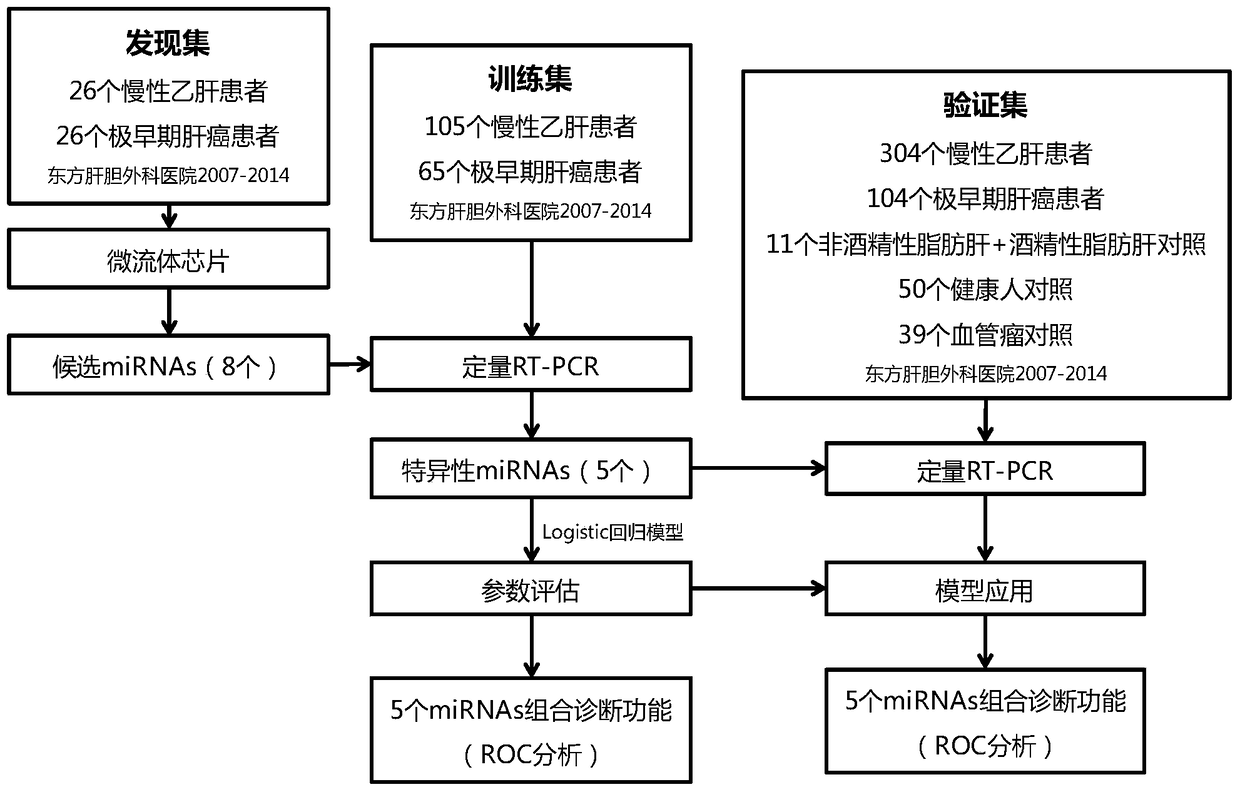
[0069] Example 1. Cohort screening of specific miRNAs molecular markers that can predict the possibility of liver cancer in high-risk groups of patients with viral hepatitis
[0070] The annual physical examination cohort of nearly 10,000 chronic HBsAg-positive patients was studied, and the serum of patients with liver cancer diagnosed half a year to one and a half years ago was collected to screen for differential expression compared with those without liver cancer miRNAs profile, screening specific miRNAs molecular markers that can predict the possibility of liver cancer in high-risk groups of patients with viral hepatitis.
[0071] The main plasma samples used in the study came from the Eastern Hepatobiliary Surgery Hospital, collected from a cohort of annual physical examinations from April 2007 to March 2014, including 9287 chronic hepatitis B surface antigen (HBsAg) positive (HBsAg-positive) patients . During the physical examination of these participants, HBsAg, alanin...
Embodiment 2
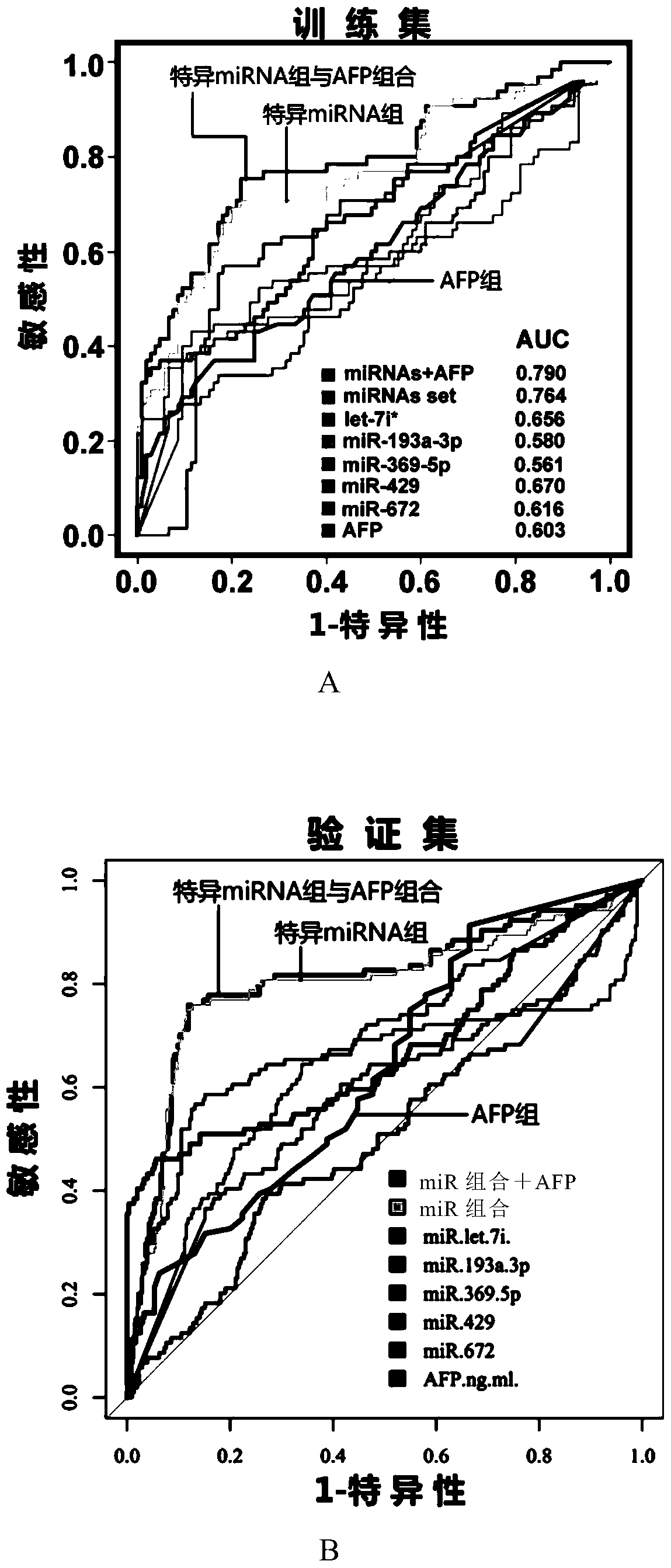
[0086] Example 2. Preparation of detection reagents and detection of training set patient serum
[0087] Based on the five miRNAs identified above, detection reagents were designed to identify early or very early liver cancer by detecting the five miRNAs.
[0088] 1. Primer design
[0089] Design the following reverse transcription primers and upstream and downstream primers and probes:
[0090] For hsa-miR-193a-3p:
[0091] Reverse transcription primer: 5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACTGGGACTTTG-3'(SEQ ID NO: 6);
[0092] Upstream primer: 5'-ACACTCCAGCTGGGAACTGGCCTACAAAGT-3' (SEQ ID NO: 7);
[0093] Downstream primer: 5'-GTACGACTCACTATAGGGACTCAACTGGTGTCGTGGAG-3' (SEQ ID NO: 8);
[0094] Probe: 5'-TTCAGTTGAGACTGGGACTTTG-3' (SEQ ID NO: 9). The probe has FAM at its 5'-end; BHQ at its 3'-end.
[0095] For hsa-miR-369-5p:
[0096] Reverse transcription primer: 5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGC GAATATAACAC-3' (SEQ ID NO: 10);
[0097] Upstream primer: 5'-AC...
Embodiment 3
[0149] Embodiment 3, prepare a kind of kit of the present invention
[0150] In this embodiment, with the detection reagent designed in Example 2, a kit comprising the following components or components was prepared: RNA extraction reagents, reverse transcription reagents for each miRNAs to be tested and internal reference, for each miRNAs to be tested and Internal reference PCR amplification upstream primers and downstream primers, RNase-free pure water, negative quality control, positive quality control.
[0151] 1, RNA extraction reagent: what used in the present embodiment is Trizol reagent (Life Technologies company, the U.S.);
[0152] 2. Reverse transcription primers, forward primers, reverse primers, oligonucleotide probes, and positive controls were synthesized by Shanghai Sangon Biotechnology Co., Ltd.;
[0153] 3. Reverse transcriptase M-MLV, reverse transcription reaction system, nucleic acid amplification enzyme and quantitative PCR reaction system were purchased...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com