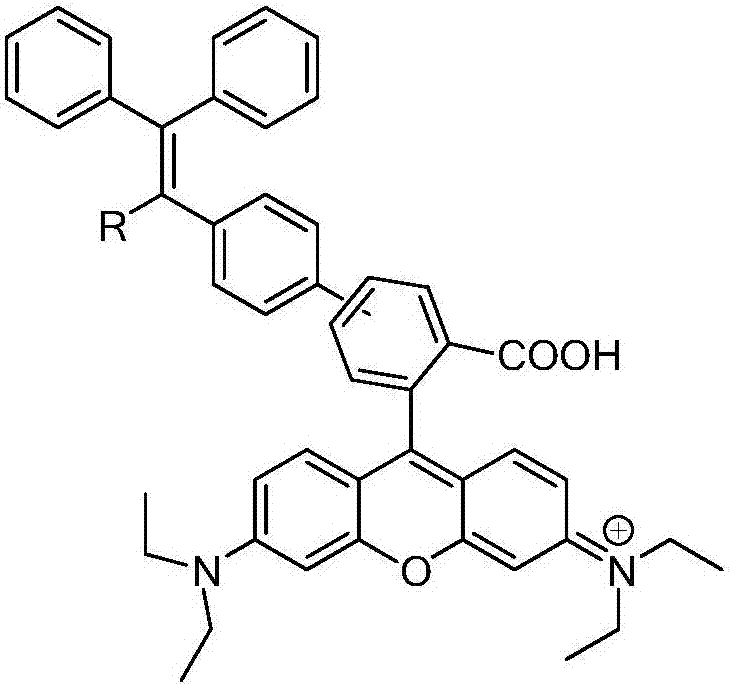
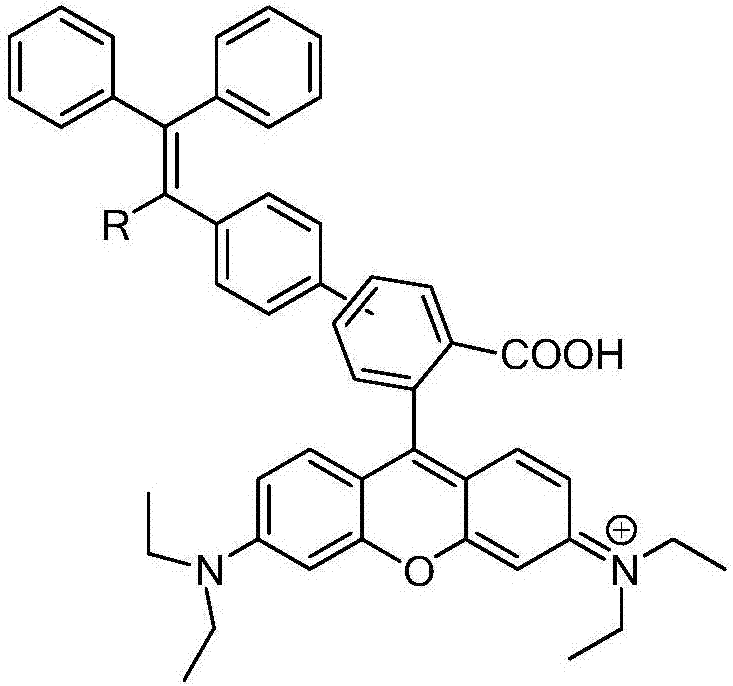
Rhodamine b derivatives with solid-state luminescent properties and their preparation and application
A technology of solid-state luminescence and derivatives, applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems that cannot be applied to living organisms, achieve the effects of reducing complexity, avoiding adsorption loss, and increasing flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Preparation method of rhodamine B derivatives with high-efficiency solid-state luminescent properties:
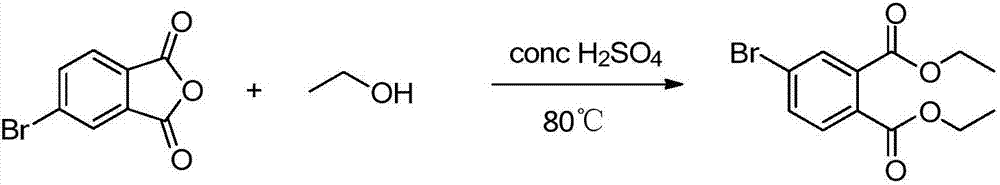
[0042] 1. Ethanol esterification protection: Add 17.62 mmol of 3-bromophthalic anhydride to 428.71 mmol of absolute ethanol, drop in 2 ml of 98% concentrated sulfuric acid, and reflux at 80°C for 8 hours under the protection of nitrogen.
[0043] Separation and purification: spin to dry the solvent, add potassium carbonate to neutralize to pH = 7, wash with water, separate the layers, extract the aqueous layer with dichloromethane, combine the organic layers, dry with anhydrous magnesium sulfate, suction filter, and spin to dry the solvent to obtain Yellow liquid product. Yield 60%.
[0044] 2. Suzuki cross-coupling: 2.32mmol of ethyl 3-bromophthalate and 2.79mmol of boric acid compound of triphenylethylene (HTPE) were added to a mixed solution of 35ml of tetrahydrofuran and 5ml of water under nitrogen protection, and 6.97mmol of Potassium carbonate and 92.98 μmol ...
Embodiment 2
[0051] Preparation method of rhodamine B derivatives with high-efficiency solid-state luminescent properties:
[0052] 1. Ethanol esterification protection: Add 17.62 mmol of 3-bromophthalic anhydride to 428.71 mmol of absolute ethanol, drop in 2 ml of 98% concentrated sulfuric acid, and reflux at 80°C for 8 hours under the protection of nitrogen.
[0053] Separation and purification: spin to dry the solvent, add potassium carbonate to neutralize to pH = 7, wash with water, separate the layers, extract the aqueous layer with dichloromethane, combine the organic layers, dry with anhydrous magnesium sulfate, suction filter, and spin to dry the solvent to obtain Yellow liquid product. Yield 60%.
[0054] 2. Suzuki cross-coupling: add 2.32mmol of ethyl 3-bromophthalate and 2.79mmol of boronic acid compound of tetraphenylethylene (TPE) to the mixed solution of 35ml tetrahydrofuran and 5ml water under the protection of nitrogen, and add 6.97mmol Potassium carbonate and 92.98 μmol ...
Embodiment 3
[0061] Preparation method of rhodamine B derivatives with high-efficiency solid-state luminescent properties:
[0062] 1. Ethanol esterification protection: Add 17.62 mmol of 3-bromophthalic anhydride to 428.71 mmol of absolute ethanol, drop in 2 ml of 98% concentrated sulfuric acid, and reflux at 80°C for 8 hours under the protection of nitrogen.
[0063] Separation and purification: spin to dry the solvent, add potassium carbonate to neutralize to pH = 7, wash with water, separate the layers, extract the aqueous layer with dichloromethane, combine the organic layers, dry with anhydrous magnesium sulfate, suction filter, and spin to dry the solvent to obtain Yellow liquid product. Yield 60%.
[0064] 2. Suzuki cross-coupling: add 2.32mmol of ethyl 3-bromophthalate and 2.79mmol of boric acid compound of triphenylcyanoacrylonitrile (TPAN) into the mixed solution of 35ml tetrahydrofuran and 5ml water under nitrogen protection, add 6.97 mmol of potassium carbonate and 92.98 μmo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com