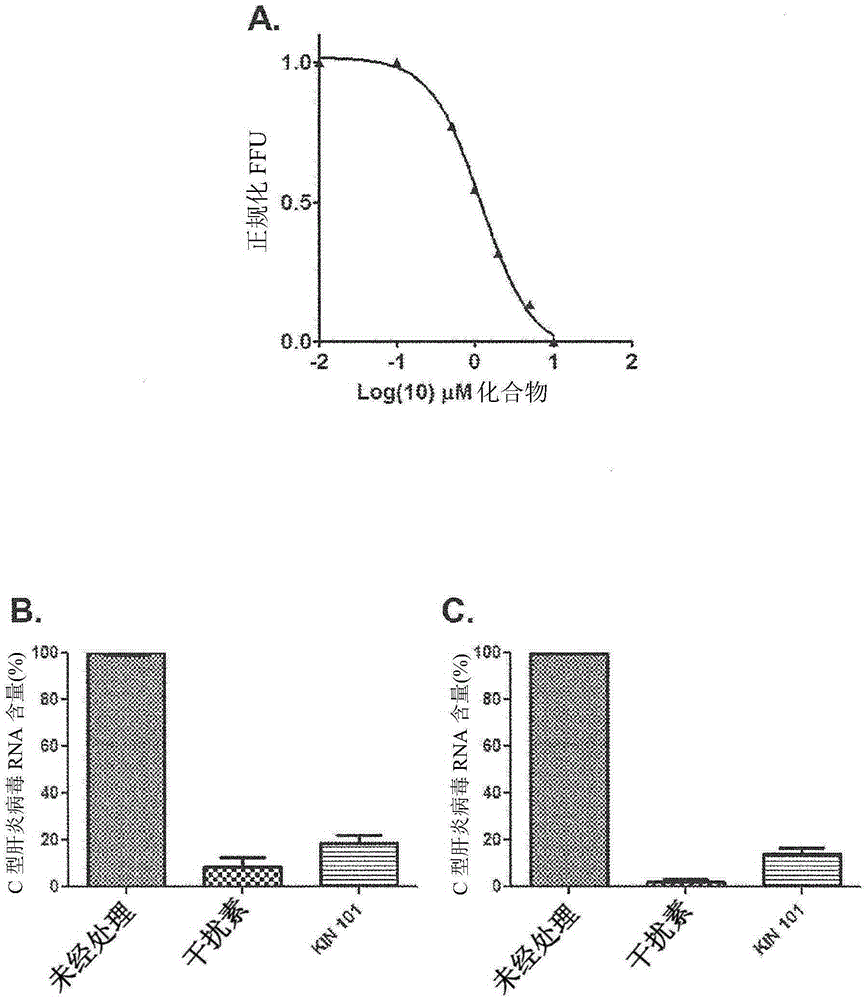
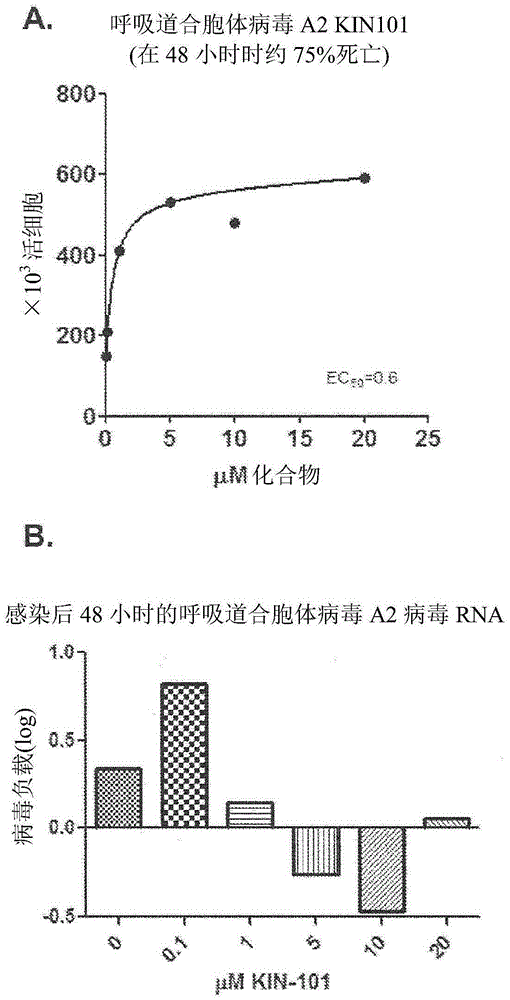
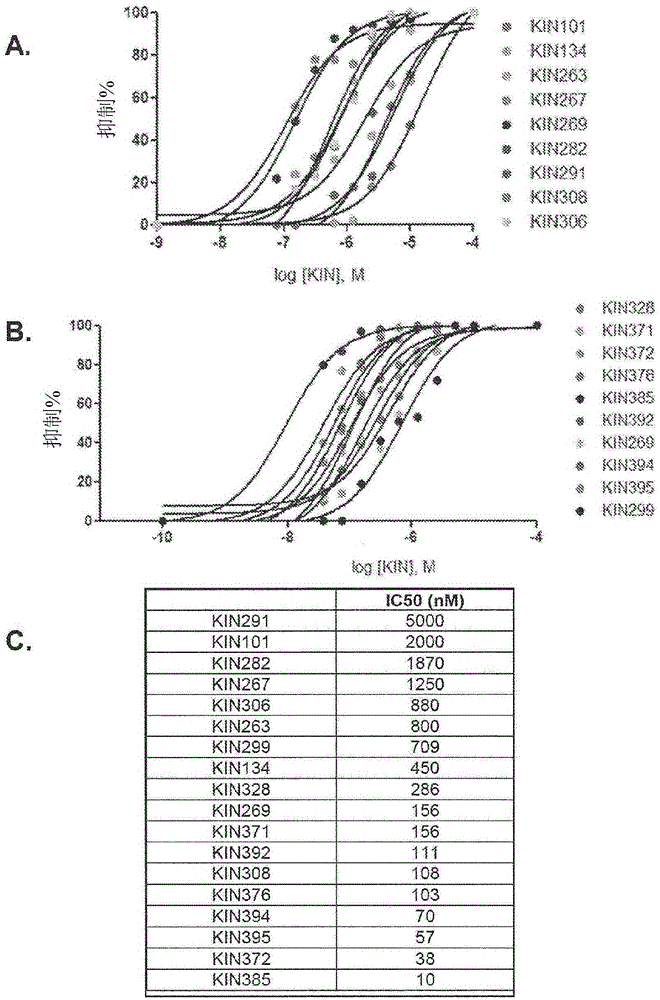
Anti-viral compounds, pharmaceutical compositions, and methods of use thereof
A compound, carbon number alkyl technology, applied in antiviral compounds, pharmaceutical compositions and its application fields, can solve the unmet needs and huge problems of effective treatment of viral infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0247] Example 1: Synthesis of compounds of the present invention
[0248] General Synthetic Scheme. Compounds of the present invention can be prepared by the methods described below in conjunction with synthetic methods well known to those skilled in the art. The starting materials used herein are commercially available or can be prepared by conventional methods known in the art (such as those disclosed in standard reference works, such as Compendium of Organic Synthetic Methods (COMPENDIUMO FORGANICSYNTHETICMETHODS), Volumes I-VI (Published by Wiley-Interscience). Preferred methods include those described below.
[0249]During any of the following synthetic sequences, it may be necessary and / or desirable to protect sensitive or reactive groups on any molecule of interest. This can be achieved by means of conventional protecting groups such as those described in: T.W. Greene, Protective Groups in Organic Chemistry, John Wiley & Sons, 1981; T.W. Greene and P.G.M. P.G.M. Wut...
example 2
[0269] Example 2. Synthesis of 3-(4-tert-butylphenyl)-4-oxo-4H-chromen-7-yl methanesulfonate
[0270]
[0271]
[0272] Step 1: Synthesis of intermediate 2-(4-tert-butylphenyl)-1-(2,4-dihydroxyphenyl)ethanone. (4-tert-butylphenyl)acetonitrile (10 g, 0.058 mol) and resorcinol (7.3 g, 0.066 mol) were added to 40 mL of BF3·Et2O and a flow of dry HCl gas was passed through the mixture overnight. The solution was then poured into 300 mL of cold water and stirred for 6 hours. The mixture was extracted with ethyl acetate and the solvent was evaporated to obtain an oil which was purified by chromatography to obtain 0.68 g of 3-(4-tert-butylphenyl)-7-hydroxy-4H-chromene-4 after chromatography - Ketones (20%).
[0273] Step 2: Synthesis of 3-(4-tert-butylphenyl)-7-hydroxy-4H-chromen-4-one. The intermediate from Step 1 (0.65 g, 2.3 mmol) was mixed with 1:1 triethyl orthoformate with anhydrous pyridine and piperidine and kept at 120-130 °C for 4 hours. The mixture was cooled and...
example 3
[0275] Synthesis of Example 3.N-[3-(4-tert-butylphenyl)-4-oxo-4H-chromen-7-yl]-N-methylmethanesulfonamide
[0276]
[0277] Step 1: Synthesis of N-(4-acetyl-3-hydroxyphenyl)methanesulfonamide. Add pyridine (1.6mL, 20mmol) to commercially available 4'-amino-2'-hydroxyacetophenone (2g, 13mmol) and methanesulfonyl chloride (1.6mL, 16mmol) in 40mL of anhydrous mixture in dichloromethane. The resulting mixture was stirred overnight at 0°C to room temperature, then diluted with dichloromethane and washed with 1M aqueous hydrochloric acid. Insoluble material appears at the interface between the two layers. The aqueous layer was back extracted twice with dichloromethane. The combined organic layers were dried over sodium sulfate, filtered and evaporated to obtain 0.11 g of sulfonamide. The insoluble material at the interface between the two extraction layers was filtered and rinsed with diethyl ether to obtain 1.3 g of sulfonamide (89% yield).
[0278] Step 2: Synthesis of N-{...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com